Planmed Verity® CBCT scanner receives FDA approval for maxillofacial imaging
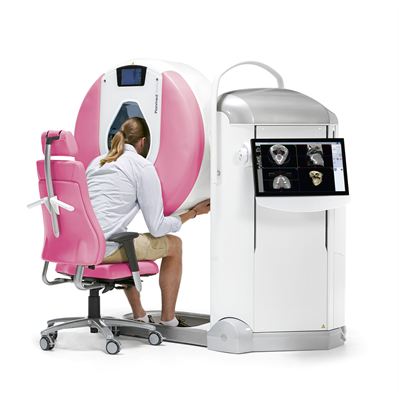
On May 14, 2015 the FDA issued an approval letter for the Planmed Verity® CBCT scanner’s MaxScan™ maxillofacial imaging option. Planmed Verity is a high-end solution for imaging of anatomies within upper and lower extremities, as well as the maxillofacial region. The system utilizes CBCT (Cone Beam Computed Tomography) technology with a flat panel detector, which provides high-resolution volumetric images. It is a compact, stand-alone unit from which all stages of the imaging process can be performed. Planmed Verity’s MaxScan imaging option enables flexible CBCT imaging of the regions encompassing the jaw and face.
Planmed Verity® has been specially designed to find subtle extremity fractures, the type most commonly missed by 2D radiographs, within the first clinic visit. The CBCT scanner’s MaxScan™ imaging option is a unique and cost-effective solution for evaluating maxillofacial fractures. It enables imaging of the jaw, teeth, sinuses, temporomandibular joints (TMJ), orbits, and airways – all at a low patient dose.
As a modern maxillofacial scanner, Planmed Verity has been designed with patient comfort in mind – ensuring a uniquely convenient imaging process. The CBCT scanner includes an adjustable, soft surfaced gantry and dedicated positioning tray, allowing for optimized patient comfort. The motorized gantry with adjustable height and tilt enables flexible positioning when imaging extremities or the maxillofacial area. A highly convenient sitting position along with an open gantry reduces patient anxiety, while lean-in positioning makes maxillofacial imaging a fast, more agreeable procedure.
“Planmed Verity is a unique imaging solution, which allows the flexible examination of seated, oblique and even standing patients”, says Mr. Vesa Mattila, Managing Director of Planmed. “We are excited about our innovative CBCT scanner now also being available for maxillofacial imaging in the United States. We are convinced that the MaxScan imaging option will be enthusiastically received, as it enables convenient capturing of high-quality images at a low patient dose.”
Planmed’s strong commitment to research and development combined with a positive focus on the future allows its products to continue to impress with exquisite design and ergonomics. In North America, Planmed products are sold, marketed and supported by the company’s US subsidiary Planmed USA, Inc. (Roselle, IL).
For further information, please contact:
Mr. Vesa Mattila,
Managing Director, Planmed Oy
Tel. +358 20 7795 301
vesa.mattila@planmed.com
Planmed Oy and Planmeca Group
Planmed Oy develops, manufactures and markets advanced imaging equipment and accessories for mammography and orthopedic imaging. Planmed's extensive mammography product range covers digital and analog units and digital breast tomosynthesis (DBT) systems, stereotactic biopsy devices, and breast positioning systems for an early detection of breast cancer. Within orthopedic 3D imaging Planmed offers low dose extremity CT imaging for quicker, easier and more accurate diagnosis at the point-of-care. Strongly committed to R&D and design, Planmed Oy exports more than 98% of its production to over 70 countries worldwide. Planmed Oy was established in 1989 and the company’s headquarters are located in Helsinki, Finland.
Planmed Oy is part of the Finland-based Planmeca Group that manufactures and markets state-of-the-art products for the medical and dental fields. The Group's turnover in 2014 was approximately EUR 740 million and it employs nearly 2,700 people worldwide.
www.planmed.com
Tags:


