BioGaia’s probiotic safe and effective in premature infants
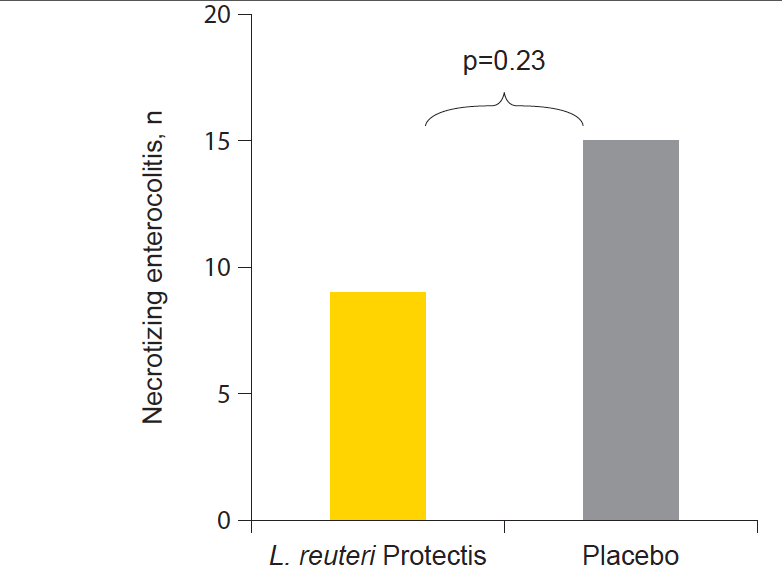
Results from the largest probiotic study to date in premature infants showed that necrotizing enterocolitis (NEC), the most common gastrointestinal cause of death and illness in premature infants, decreased by 40% in the infants supplemented by Lactobacillus reuteri Protectis compared to placebo. Further, in the infants below 1500 grams Lactobacillus reuteri Protectis reduced episodes of feeding intolerance by 43%.
The safety of Lactobacillus reuteri Protectis in this high-risk population was also confirmed, but there was no significant difference between the groups on the primary outcome, frequency of death or hospital acquired infection.
“Although our study was under-powered to show significant differences in the primary outcome, the trends are consistent with those observed in meta analyses on NEC and death”, says Professor Mario A. Rojas, Department of Pediatrics, Wake Forest School of Medicine, Winston-Salem NC, United States.
“Unfortunately the study was terminated early, but despite this it is now confirmed that treatment of premature infants with Lactobacillus reuteri Protectis is both safe and clinically relevant”, says Peter Rothschild, Chief Executive Officer at BioGaia.
Largest probiotic study in premature infants
A total of 751 infants were included in the study, which was considerably less than the number planned (1110) and required to reach significance in the outcome parameters. The study was terminated early, which was related to substantial drops in recruitment rate and funding restrictions from the independent funding institute.
In the multi-centre, double-blind, randomised, placebo-controlled trial in nine neonatal intensive care units in Colombia, infants born prematurely, with a birth weight of 2000 grams or smaller, were randomized to two groups, one that was given five drops daily of Lactobacillus reuteri Protectis (BioGaia ProTectis, n=372) and one that was given a corresponding placebo (n=378).
The study was published online in Pediatrics on 15 October 2012.
Necrotizing enterocolitis – a fatal disease
NEC is the death of intestinal tissue. It predominantly affects premature infants and often results in death or serious medical or neurodevelopmental complications, such as cerebral palsy (CP) and cognitive, visual or hearing impairment. The rate of NEC is highest in the smallest neonates (< 1500 grams) where around 10% of the infants are infected. The death rate ranges between 20 and 30%, with the highest rate among infants requiring surgery.
Lactobacillus reuteri – a well researched probiotic
Lactobacillus reuteri is one of the world's most well researched probiotics, especially in young children. To date 92 clinical studies using BioGaia's human strains of Lactobacillus reuteri have been performed in more than 7,700 individuals of all ages. Half of the studies have been performed in premature babies, infants and children. Results are published in 63 articles in scientific journals (September 2012).
Latest press releases from BioGaia
2012-10-12 BioGaia signs exclusive distribution agreement for its probiotic drops and tablets in Pakistan
2012-09-17 Lactobacillus reuteri Protectis shown to reduce colic in infants
2012-09-07 BioGaia’s probiotic prevented necrotizing enterocolitis in high risk premature infants
BioGaia has published this information in accordance with the Swedish Securities Market Act. The information was issued for publication on 15 October 2012, 15:30 am CET.
For additional information please contact
Peter Rothschild, President, BioGaia: +46 8 555 293 00
Mario A. Rojas, Dr, Department of Pediatrics, Wake Forest School of Medicine: mrojas@wakehealth.edu
Eamonn Connolly, Senior Vice President Research, BioGaia: +46 8 555 293 00
BioGaia is a healthcare company that develops, markets and sells probiotic products with documented health benefits. The products are primarily based on the lactic acid bacterium Lactobacillus reuteri, which has probiotic, health-enhancing effects. The class B share of the Parent Company BioGaia AB is quoted on the Mid Cap list of the NASDAQ OMX Nordic Exchange Stockholm. www.biogaia.com


