CS MEDICA A/S Announces “Proof of Concept” with Successful Clinical Trial Results of CANNASEN® Pain Patch
CS MEDICA A/S (“CS MEDIA” or “The Company”), a leading MedTech company specializing in cannabis and pain management, is pleased to announce the successful completion of its clinical trial for CANNASEN® Pain Patch
In the clinical trial, CANNASEN® Pain Patch treatment was associated with significant reductions in pain intensity and was well tolerated by all patients in the treatment group. The reduction of pain was measured by the widely used WOMAC1-Pain Index, a questionnaire and index for assessing pain, stiffness, and physical function in individuals with joint degeneration and inflammation.
Lone Henriksen, CEO & CSO of CS MEDICA comments;
"We are thrilled with the positive results of this clinical trial, which confirms the efficacy, safety, and tolerability of the CANNASEN® Pain Patch in managing pain. This is a significant milestone for our company, and it truly supports our go-to-market plans for our innovative and effective pain management solution."
Brief Study Summary
The purpose of the trial was to determine whether the use of the CANNASEN® Pain Patch reduces subject pain as measured by subject pain scores, WOMAC[1]- scores (pain, stiffness, and physical function), global assessment scores.
| Study design Study Type: |
Clinical Trial - Post Market Clinical Follow-up (PMCF) |
| Actual Enrolment: | 80 participants |
| Intervention Model Description: | single-arm, open-label study |
| Official Title: | Post Market Clinical Follow-up (PMCF), Musculoskeletal Pain |
| Estimated publication time: | Q4, 2023/Q1 2024 |
Approximately 80 subjects who met the trial entry criteria were enrolled at one investigative site. The subjects were assigned to receive CANNASEN Pain Patch, which they applied topically for the duration of 24 hours. The maximum trial duration for each subject was 24 hours and included a screening period. At the end of the screening period, eligible subjects were assigned to the trial treatment on Day 1 (Visit 1/Baseline) of the treatment period.

The trial, a Post Market Clinical Follow-up (PMCF), single-arm, open-label study, evaluated the efficacy, safety, and tolerability of CANNASEN® Pain Patch when applied for up to 24 hours to adults between the ages of 18-65 years.
During the treatment period, compliance with the treatment regimen, concomitant medications, and adverse events (AEs) was assessed. The investigator scored the disease severity of the subject using the Subject Global Assessment, Pain-Visual Analogue Scale (VAS)[2], WOMAC- Index for Pain. The subjects were asked to complete the EuroEQ-5D-5L five dimensions (EQ-5D) (EQ-5L)[3] and Treatment Convenience Scale questionnaires[4]. Safety assessments, including local skin reaction, AEs, laboratory tests, vital signs, and physical examinations were performed.
The results of the trial were highly encouraging. CANNASEN® Pain Patch showed significant efficacy in reducing muscle and joint pain, swelling, and stiffness while improving mobility and quality of life. Moreover, the after-use long-lasting effect of the patch was also observed. The results are illustrated by the WOMAC Pain index below.
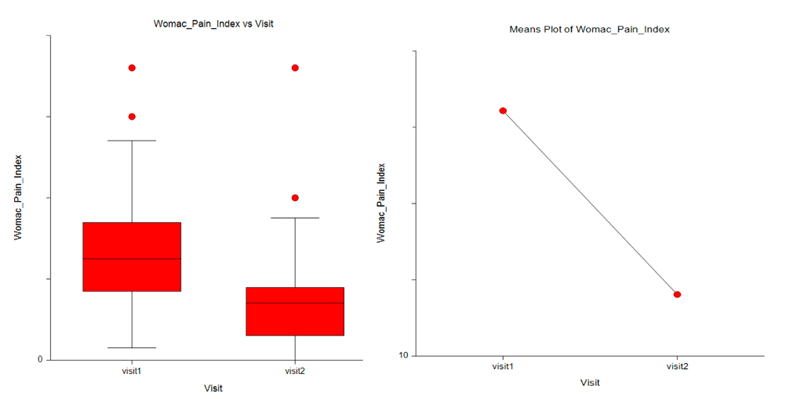
Results
The full study with the results of each primary and secondary efficacy endpoints will be published in a scientific paper on a later stage, hence we cannot disclose the full trial and efficacy endpoints.
CANNASEN® Pain Patch is a unique, transdermal patch infused with CBD that offers a targeted approach to pain management. The patch delivers a controlled dose of treatment directly to the affected area, providing long-lasting pain relief without any side effects commonly associated with oral pain medications. CANNASEN® Pain Patch is available as an over-the-counter (non-prescription) treatment for the temporary relief of minor aches and pains in muscles and joints.
Lone Henriksen, CEO & CSO of CS MEDICA continues;
“Our successful, clinically proven, and documented results will help us speed up our go-to-market plans and begin taking market shares of a growing market. It also means we will look into a broader range of patches to match the demand for pain relief with CBD benefits.”
The pain patch market, is valued at 316,8 million in 2022 and is expected to grow with a CAGR of 13,6% up to 2030[5]. National Center for Biotechnology Information (NCBI) estimates that 20% of adults suffer from pain globally and 10% are newly diagnosed with chronic pain each year[6]. Moreover, interest in alternative treatments continues to increase and the focus on CBD's potential analgesic properties gains momentum. Based on the positive results from this clinical trial, CS MEDICA anticipates that the potential for CANNASEN® Pain Patch is compelling.

[1] Western Ontario and McMaster Universities Arthritis Index (WOMAC) is widely used in the evaluation of Hip and Knee Osteoarthritis. It is a self-administered questionnaire consisting of 24 items divided into 3 subscales:
- Pain (5 items): during walking, using stairs, in bed, sitting or lying, and standing upright
- Stiffness (2 items): after first waking and later in the day
- Physical Function (17 items): using stairs, rising from sitting, standing, bending, walking, getting in / out of a car, shopping, putting on / taking off socks, rising from bed, lying in bed, getting in / out of bath, sitting, getting on / off toilet, heavy domestic duties, light domestic duties
WOMAC Index was developed in 1982 at Western Ontario and McMaster Universities. WOMAC is available in over 65 languages and has been linguistically validated.
[2] The Global Assessment is a subjective measure of overall pain perception, often rated on a scale from "no pain" to "worst possible pain." The Pain-Visual Analogue Scale (VAS) is a commonly used method for assessing pain intensity on a continuous scale, where the individual marks their current pain intensity on a line with anchor points representing extremes of pain intensity. Both measures are widely used for evaluating pain severity in clinical and research settings.
[3] The EQ-5D-5L is a health-related quality of life measure that assesses five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. It has five response options for each dimension and generates an overall health state description and index score. It is widely used in health outcomes research and clinical practice.
[4] Treatment Convenience Scale questionnaires are assessment tools use to measure how convenient or burdensome an medical treatment is perceived to be from the patient’s perspective, They capture aspects such as ease of administration, dosing frequency, and impact on daily activities, They are used in research and clinical practice to inform treatment decision-making and patient adherence.
[5] https://www.thebrainyinsights.com/report/cbd-patch-market-13159
[6] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3201926/
For more information about CS MEDICA, please contact:
Lone Henriksen, CEO
Phone: + (45) 71 20 30 47
Email: lh@cs-medica.com
Website: https://www.cs-medica.com/
CS MEDICA A/S is a Danish-based MedTech company operating within research, development, manufacturing, commercializing, and a part of the pharmaceutical industry. The company combines science and nature with the purpose of creating products for a better every day by using modern technology to research and utilize different compounds found in the cannabis plant. CS MEDICA offers efficient, safe OTC alternative treatments for autoimmune and stress-related disorders under the trademark CANNASEN® or own label options.
The company is listed on Spotlight Stock Market in Stockholm (symbol: “CSMED”). more information, visit cs-medica.com, and LinkedIn.
Tags:




