CS MEDICA A/S presents CEO Letter of December 2022
The year is coming to an end, and it has been an intense but successful business year, despite the negative macro events and criticism that have characterized 2022. December continued to be intense as we entered the final steps of our patent processes for our CBD technology, finalized shipments for our new customers, and also started up production with a new packaging design and updated compliance terms to match the MDR transitioning.

HAPPY NEW YEAR 2023
We are proud of our 2022 deliverables, and the team and new partners are hungry for more growth and success in 2023. We believe we’ll launch new countries, products, and channels. We’ll grow the brand and customer pool as we increasingly become data-driven and digital to match the global market needs.
Before we enter the New Year, let’s look at some of the achievements and learnings we bring with us from 2022.
HIGHLIGHTS FROM 2022
The need for a new Communication Strategy
The priority for the last six months has been to ensure the company’s ‘engine’ is as efficient and growth-minded as possible. As a result, the organization has consolidated, new processes and strategies implemented, and partnerships with new global distributors created. Now it is time to reposition the company to its DNA, hence communicate and act as an IPO company with the proper equity story.
Most perceive us as a CBD company within cosmetic or oil products, not a company that has researched and developed medical device products with the intended purpose to treat and improve autoimmune and stress-related diseases. The low understanding of us being a first mover, operating under the pharmaceutical legislation with cannabinoids as an ingredient, is what we need to approach.
Consequently, we are mainly measured by revenue, not the patents, clinical findings, and the R&D we deliver. We are often criticized for our burn rate, for the ambitions we communicate, and for a maybe too transparent information flow. But we hear, learn, and act – highly appreciating the feedback we get from our stakeholders.
It means we have said goodbye to our previous advisors and signed with new ones who understand that CS MEDICA is a MedTech company with a unique CBD technology. We cover both Research and Development under the pharmaceutical legislation, sometimes causing long lead times to market due to high demands for tests, clinical trials, MDD/MDR processes, patents, and local registrations. AND we're a commercial unit with finished products which we Produce, Sell, Distribute, and Brand. Not to forget, we are presently registered, as far as we know, as the only global company with topical and intranasal treatment products as medical devices with cannabinoids.
So, we will implement a new communication strategy in January 2023.
The latest news on Patents and CBD Technology
All patent applications are continuations of the patent application earlier submitted. All conducted according to plan and process. Please find more information about status on patents here.
MDR transitioning on track
Our CANNASEN® products were filed and launched as Class I under the MDD (Medical Device Directive) before May 26, 2021, the date on which the MDD was replaced by the more restrictive MDR (Medical Device Regulation) (EU) 2017/745.35. With the new MDR, four of our treatment products are to be lifted to a Class IIa. However, with in a transition period up to May 2024, permission has been granted for products to remain on the market, if they were filed and launched under MDD before the change in legislation. This means new entries to the market will have to launch directly under MDR (similar to FDA) and probably start last in the long queues at Notified Bodies, the organizations designated to assess the conformity of certain products before being placed on the market. The pressure is now so high, the EU has proposed the transition period to be extended by additionally 4 years, up to 2028[1],
As we are already adopting the requirements following MDR and preparing the classification lift, our products are allowed to stay at the marked during the transitions period and after the transition period provided that the extended requirements and the classification lift are finalized.
We are presently, as far as we know, as the only global company products, containing cannabinoids regulated under MDR[2]. With the proposed extension of the MDR transition period, we gain additional years alone on the market; a significant competitive advantage, especially if the Notify Bodies first assess medical devices launched before May 2021, blogging[3] for new cannabinoid products to be introduced on the market under MDR, as they will be at the back of the assessment queue, currently corresponding to 11,5 years head start.
Despite the discussion about potentially prolonging the MDR transition period, we plan to meet the current deadline of May 20241 for assessment at the Notify Body, and we are currently in the queue to become a client at/assessed by the Notify Body. We expect to be ready to send the applications in H1 2023, for those medical device products moving to class IIa according to the new MDR classification system:
- CANNASEN® Arthritis Gel
- CANNASEN® Wound Gel
- CANNASEN® Protective Nasal Gel
- CANNASEN® Nasal Spray Night
The CANNASEN® Pain Patch and CANNASEN® Psoriasis Gel will stay under class I according to the new MDR classification system, and these two products are subject to a self-notification. However, the technical files will be updated according to MDR on both products incl. clinical trials, clinical evaluation, and safety data.
Harmonizing production
To increase production efficiency and reduce costs as the lead time to market, we have harmonized the product elements, such as paper boxes and tubes, staying within compliance. We have also implemented a new packaging design to strengthen brand hierarchy and new processes for customers’ own label production.
New Product Pipeline consolidated based on market learnings
In 2022 we launched CANNASEN®’s Pain Patch, Wound Gel, Protective Nasal Gel, Nasal Spray Night, and Psor+Atopic Lotion on a global scale, reaching a relatively broad range of 9 finished products on the markets (6 medical devices and 3 cosmetic products, all topical and intranasal). The learnings from consumers and our partners are that the product portfolio is perceived as CBD products covering many different unrelated categories.
As we combine science, nature, and innovation in our products to make a difference, we have chosen to reframe our brand hierarchy and portfolio structure to provide transparency. By implementing a branded house strategy, we provide alternative healthcare treatments and solutions to patients within the categories we cover, with cannabinoids as a uniquely beneficial ingredient, non THC.
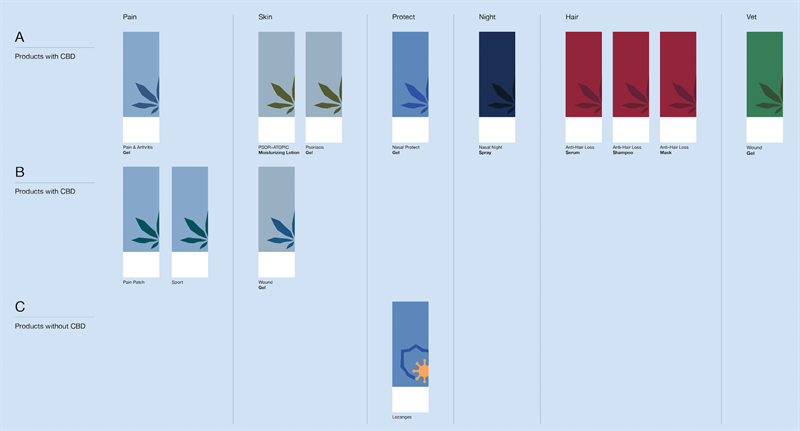
CANNASEN® Categories Color System
We consolidate and focus on our portfolio to prioritize the MDR transitioning and leverage existing products. We investigate revenue options for entering new categories and markets with the same formulas but new trials, sizes, and designs.
The VET portfolio is a good example. The category is significant when we look at the need for pain relief, stress, and wound healing. We evaluate if we can also implement this growth strategy to enter the Sports category.
The VET products from within the portfolio
Based on a dialog with a European-based veterinarian company, and recently also a US-based company within the VET industry, our vet product line is pulled forward in timing. We have considered the needs of veterinarians and the animals they care for. The project steps we have initiated and conducted are:
- Market Research: Mapped available products and identify gaps or areas for improvement. The target audience for our product (e.g., small animal practitioners, large animal practitioners, DtC) has been concluded to be dogs, cats, and horses.
- Product purpose: Defined the problem and what needs to address. It has been the guide in the development process.
- User research: We conducted the study with veterinarians and other relevant professionals to gather insights and feedback on the product idea. It helped us refine the design and size of the product to ensure it meets the needs of the intended users.
- Develop prototypes: Using the feedback from veterinarians to redesign and create product prototypes. It allows us to test the product in a controlled setting and gather further feedback.
- Seek regulatory approval: Depending on the nature of the product and markets, we may need to seek regulatory approval before being launched. This process may involve submitting the product for testing and demonstrating its safety and effectiveness.
As we are finalizing the VET products' artwork and investigating the regulatory need for approval, we estimate to be able to cover the market requests during 2023.
Accelerating growth despite long G2M lead time
Based on our learnings, we must acknowledge the lead time to market on medical devices under the pharmaceutical legislation is longer than expected. The global demand is high, and thus we’re registered in Europe, the UK and hold free sales certificates in 160 countries outside the EU; we still see local registrations vary from 1 to 12 months. Then the production lead time is to be added with additional 1-4 months depending on the product and localization.
To optimize the sales pipeline and speed to market, we have reframed our sales strategy to prioritize countries with shorter registration processes and, additionally, a relatively high search/education on CBD as an ingredient. Equally, we focus on gaining distributional partnerships to support us in growing reach and volume, as a team of partners helps us grow faster than our organization can do alone. Additionally, new partners provide new territories and knowledge of local needs.
By leveraging our portfolio, we also expand our focus on shorter-term revenue streams to ensure a faster sales turnover and profitability. We’ll evaluate our business plan and NPDs to optimize this approach.
Increasing repurchase customer pool – BtB & DtC
We are delighted to see our customers returning for additional orders increases since the reoccurring business model is a crucial part of our business and long-term partnership strategy but also proof of concept. On our own label customers' re-orders, we also see product line extensions besides repurchases of the existing portfolio.
Launching on the most essential AMAZON platforms in Europe
After reframing the strategy for Amazon and shifting agency support, we have updated the entire backend and brand store in Germany and Sweden. We are also launching at Amazon France and Italy as we speak, as in Spain with selected products. Furthermore, by securing a logistical setup for the UK via our partner Cleanpure UK Ltd., we’re launching all products and a brand store on Amazon UK before the end of 2022.
Controlling burn rate and reframing the need for liquidity
Like other MedTech companies, CS MEDICA's investment in research and development, production, sales, marketing, etc., is heavy for a start-up. Therefore, adding an IPO could seem questionable due to additional costs and the organization's readiness. Nevertheless, highlighting the company's seriousness and gaining trust was essential, and an IPO was the right path in 2021 to fund the company's R&D, patents, and market growth.
As we operate within the entire value chain, combined with an IPO, we currently track a high burn rate for our size, but not compared to the industry MedTech benchmark. Thus, with a funding strategy focusing on securing smaller funding rounds, planning to follow increased valuation, the company is left with continuous liquidity challenges due to negative macro events.
To control the burn rate, we have gone through a major consolidation process in 2021 /2022, focusing on optimizing the sales department and processes, making the organization more agile and efficient for the new requirements under MDR. The consolidation process is at its final stages, and parallel with the company's revenue growth, the burn rate has been reduced.
CS MEDICA is adjusting its funding strategy to secure further funding, maximizing the company's first-mover advantages through rapid global rollout.
Looking back at the funding efforts
CS MEDICA's shares were admitted to trading on the Spotlight Stock Market in Denmark on 14. September 2021. The Spotlight Market is a multilateral trading facility ("MTF") governed by a less extensive rulebook than the Main Market.
While planning the IPO, we chose to secure funding in smaller rounds rather than a single large round. The strategy was based on the following considerations:
- Control: By raising funding in smaller rounds, we maintain more control over CS MEDICA's equity and financing terms.
- Valuation: We wanted to secure funding at the highest possible valuation. By raising funding in smaller rounds, we expected to secure a higher valuation in each round, giving us time to demonstrate progress and traction to investors.
- Flexibility: Raising funding in smaller rounds gives us flexibility regarding how we use the funds, focusing on specific projects.
- Dilution: By raising funding in smaller rounds, we minimize the dilution of the shares.
At the IPO, we followed the Swedish method, offering units instead of shares, where one unit consisted of 5 shares at a share price of 7,70 DKK, combined with two warrants one year after the IPO, with an exercise price of +20% (9,30 DKK). The IPO of units was oversubscribed with a subscription ratio of 158%, securing 22.3 MDKK in funding, before the costs of the IPO. Since the IPO CS MEDICA secured one direct Issue of 850 TDKK in February 2022 at a share price of 8,5 DKK, followed by two warrants exercises in August and September 2022, of total 12,5 MDKK[4], at share prices of respectively 9.3 DKK (TO1) and 10.3 DKK (TO2). The TO1 was accomplished, against all odds, with a 92% succession. A larger part of the funding covered a complimented bridge loan of 6 MDKK made in May 2022 due to a delay in clinical trials hence revenue, caused by COVID-19.
New funding strategy
We are currently adjusting our funding strategy to embrace the structure of our budget, which deviates from the traditional linear and organic growth curve in its form and execution. Furthermore, we aim to establish a 2 to 3-tier funding strategy that ensures we keep momentum, utilize the competitive gap in the market we have, and deliver to our shareholders. Besides placing us in a position where we can decline funding offers that don't reach acceptable terms for the company and shareholders.
To secure optimal choices and adoption, we are currently evaluating different financial advisors to secure the necessary assistance. The financial advisor will also play a crucial role in ensuring our long-term market lift goal, to secure higher liquidity[5] and visibility[6] in the CS MEDICA share.
CS MEDICA's admission to trading on the Main Market is subject to an extensive application process, and the company must meet several objectives before the listing, meaning this is a long-term goal for the company. One of the more technical requirements is using IFRS standards in the annual accounts. CS MEDICA has thus decided to postpone the publication of the Annual Accounts for 2021/2022 to have the necessary time to adopt the IFRS already in the upcoming annual accounts.
The financial advisor is also expected to assist with the right network opportunities, risk management, and valuable insights in general. CS MEDICA has now included TOP-tier assistance from the start-up industry in Denmark to secure the process.
We expect to sign with new financial advisors in January 2023.
Negotiations with Inner Mongolia Rong Shi Hi-Tech Co.("RongShi") are now awaiting Signatures at an in-person meeting
As part of the G2M strategy, we nurture our close partners to invest in our vision, ambitions, and company growth plans. Especially if we see benefits to strengthening our alliance and speed to market.
On the 25th of September 2022, CS MEDICA initiated negotiations on a Direct Issue with Inner Mongolia Rong Shi Hi-Tech Co., Ltd in connection with the company's entering the Asian market. The potential Investment was supplemented by an interest in setting up a joint venture ("Joint Venture") that should facilitate the production and delivery of the CANNASEN® or as preferred, a local branded product line to the Asian market.
From the beginning, the share price was agreed at 31,50 DKK per share. However, following the dilution after the TO1 and TO2 warrants in August and September 2022, the Share price was subsequently adjusted to 28,13 DKK per share.
The due diligence, Investment, and the Joint Venture agreement are drafted. CS MEDICA and RongShi are currently preparing to finalize the contracts at a meeting in Denmark or China. Unfortunately, the key persons from RongShi have been infected by COVID in December, consequently, the meeting arrangements planned for December are currently on hold.
We look forward to updating the market in January
[2] Legal over-the-counter (OTC) cannabinoid products only include cosmetics and Medical Devices delivered Topically and intranasally. The European Medicines Agency EMA, the UK and now Hong Kong have currently initiated a withdrawal of all oral CBD oils and other CBD supplements. This directly results in a large portion of the current CBD products being removed from the market leaving only authorized Medical Devices and cosmetics products. As these two segments are the main focuses of the CANNASEN® brand, we believe that the change in the law is in our favour.
[3] Medical devices launched before May 2021 accounts 23.000 devices for assessment before the MDR deadline. Currently, only 2.000 products have been assessed by a Notify Body since the MDR implementation in 2017, meaning the present queue for assessment will, with the current assessment speed, take 11.5 years to complete.
[4] before costs.
[5] Liquidity: Main stock exchanges typically have higher liquidity than MTFs, which means that it is easier for investors to buy and sell shares of a company listed on a main exchange. This can make it easier for the company to raise capital and can also make the company's shares more attractive to investors.
[6] Visibility: Being listed on a main stock exchange can increase the visibility and credibility of a company. This can make it easier for the company to attract investment and can also make it more attractive to potential customers and partners.
For more information about CS MEDICA, please contact:
Lone Henriksen, CEO
Phone: + (45) 71 20 30 47
Email: lh@cs-medica.com
Website: https://www.cs-medica.com/
CS MEDICA A/S is a Danish-based MedTech company committed to improving people's lives with products that make a difference. We combine science, nature, and passion to deliver innovative alternatives with high efficacy and bioavailability to patients. From autoimmune to stress therapies, we aim to transform healthcare with more effective treatments.
We research, develop, manufacture, commercialize and brand over-the-counter (OTC) products under pharmaceutical legislation. Currently, 21 products with cannabidiol aimed at autoimmune and stress-related diseases have been developed. The first products reached Danish stores in 2020, and now 9 products are available on the European market at more than 500 points of sale. Another 12 products will be launched before the end of 2024, with 11 patent-pending products. The product line surpasses competing products on the market by being located at the intersection of natural products, science, and cannabinoid technology while being registered under pharmaceutical legislation.
CS MEDICA was recognized earlier this year as a company of Europe's top 10 MedTech companies at European Lifestars Awards as the company both Research & Develop, Manufacture, Distribute and Brand life-supporting medical technologies.
Tags:



