Diamyd Medical plans first interim report from the study DIAGNODE-1 where the diabetes vaccine Diamyd® is administered directly into lymph nodes
Diamyd Medical (Nasdaq Stockholm First North, Ticker: DMYD B) today announced in its first quarterly report for the fiscal year 2015/2016 that a first six month interim report for DIAGNODE-1, a clinical pilot study where the diabetes vaccine Diamyd® is administered directly into the lymph node, is intended to be presented during the first quarter of 2016. The study is open labeled, meaning not placebo-controlled. Professor Johnny Ludvigsson, Linköping University, is principal investigator and sponsor of the study. The report also announced that pre-clinical testing is ongoing to produce antigen presenting cells with the aim to be used in cell therapy treatment.
Moreover, the CEO's comments in today's quarterly report reads as follows.
We take great pleasure in reporting that Diamyd Medical continues to strengthen its position as a world leader in the development of Antigen Based Therapy for type 1 diabetes. A positive trend for the Diamyd® therapy could be reported from the end of the first 15-month period of the ongoing 30-month DIABGAD study. Limited six-month findings from the EDCR study will soon be available, as well as limited six-month findings from the DIAGNODE study. Particularly pleasing is the growing interest shown by the pharmaceutical industry in our approach to combining GABA with antigen-based therapy for autoimmune diseases, not limited to type 1 diabetes, and for certain inflammatory disorders.
A complete cure for type 1 diabetes could be expected to include: A) manipulation of the immune response to facilitate susceptibility to tolerance induction; B) introduction of immunological tolerance to the specific antigens exposed to the autoimmune attack, and C) increasing the functional beta cell mass to enable sufficient endogenous insulin secretion.
A possible scenario is that, while several companies may develop A and C components, Diamyd Medical will be first to market with a B component, and then continue to further improve on its antigen-specific therapy.
We are very excited about being the leading company in terms of component B development, i.e. the induction of tolerance to specific beta cell antigens. Several clinical trials have been carried out with GAD65 as the beta cell antigen. In a Phase III trial, the diabetes vaccine Diamyd® (GAD in alum) demonstrated 16 percent (p=0.1) higher efficacy than a placebo in terms of the ability to produce insulin after 15 months. Diamyd® has demonstrated a good safety profile in trials with more than 1,000 patients.
We believe that our B medication component (the diabetes vaccine Diamyd®) can be granted market approval as soon as the tolerance-inducing effect of the diabetes vaccine can be enhanced, preferably through a combination with an existing and market-aproved A component. Think cancer – a combination of medications usually provides the best treatment outcomes.
There is a steadily growing stream of possible A and C medications that through non-specific mechanisms could enhance the tolerance-inducing ability of antigen-specific therapy for type 1 diabetes. Most of these could in principle be used in combination with the diabetes vaccine Diamyd®. Examples of such molecules include GLP-1 analogues, DPP4 inhibitors, metformin, granulocyte colony stimulating factor (GCSF), antithymocyte globulin (ATG), abatacept, alefacept, demethylation agents, the IL-10 cytokine, ustekinumab (Stelara) and antibodies against T and B blood cells. We have chosen to study Diamyd® in combination with some A components, such as ibuprofen, etanercept, vitamin D and GABA (the last-mentioned for which, as previously reported by Diamyd Medical, interesting pre-clinical proof of concept has been shown, see the figure below).
Although our antigen-specific drug candidate, Diamyd®, is the leader in its class, and could be approved as a separate medication in combination with a suitable A component, a potential Diamyd 2.0 is currently under development, in where additional antigens such as gliadin and/or proinsulin may be introduced to broaden the patient population, in which Diamyd®, can possibly stop the autoimmune attack on beta cells.
We are also studying the effect of various administration methods for the diabetes vaccine Diamyd®, such as subcutaneous injection in the arm, subcutaneous injection in the abdomen (closer to the pancreatic draining lymph nodes) and directly into lymph nodes. Pre-clinical testing is ongoing with the ex-vivo co-culturing of antigens and tolerant antigen-presenting cells (APCs), with the goal of gradually injecting them back into patients (cell therapy).
Once the autoimmune attack has been overcome, the beta cell mass must be increased so that individuals with type 1 diabetes can produce enough insulin on their own. There are several strategies for such C components: transplantation of insulin-producing cells combined with immunosuppression to prevent rejection; transplantation of insulin-producing cells that are encapsulated to avoid being recognized as foreign tissue; autologous transplantation of stem cell-derived beta cells, and administration of beta cell regeneration components, such as e.g. GABA molecules.
Current development pipeline - Pre-clinical proof-of-concept
Combination therapy using GABA and antigen (GAD65) SELECTED RESULTS
GABA + GAD/alum combination therapy shows significant synergistic effects on normoglycemia in NOD mice
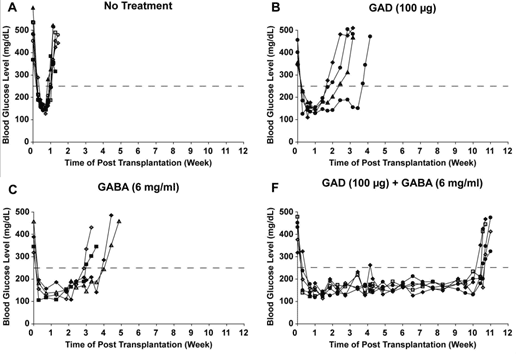
REFERENCES: Tian, Jide, Hoa Dang, and Daniel L. Kaufman. "Combining Antigen-Based Therapy with GABA Treatment Synergistically Prolongs Survival of Transplanted ß-Cells in Diabetic NOD Mice." PLoS ONE, 2011.
Current development pipeline - Pre-clinical proof-of-concept
Combination therapy using GABA and antigen (Proinsulin) SELECTED RESULTS
GABA + Proinsulin/alum combination therapy shows significant synergistic effects on normoglycemia in NOD mice
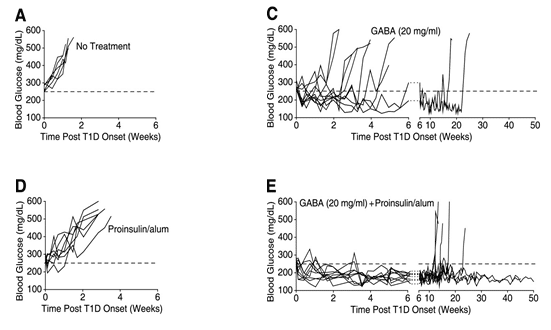
REFERENCES: Tian, J., H. Dang, A. V. Nguyen, Z. Chen, and D. L. Kaufman. "Combined Therapy With GABA and Proinsulin/Alum Acts Synergistically to Restore Long-term Normoglycemia by Modulating T-Cell Autoimmunity and Promoting -Cell Replication in Newly Diabetic NOD Mice." Diabetes, 2014, 3128-134.
A new calendar year has begun. One thing is certain – it will be an eventful year. We are looking forward to working hard – with the goal of generating value for our shareholders, and for those with type 1 diabetes.
About Diamyd Medical
Diamyd Medical is dedicated to finding a cure for autoimmune diabetes through pharmaceutical development and investments in stem cell and medical technology.
Diamyd Medical develops the diabetes vaccine Diamyd®, an Antigen Based Therapy (ABT) based on the exclusively licensed GAD-molecule. The Company’s licensed technologies for GABA and Gliadin have also potential to become key pieces of the puzzle of a future solution to prevent, treat or cure autoimmune diabetes, and also certain inflammatory diseases. At this time six clinical studies are ongoing. Diamyd Medical is one of the major shareholders in the stem cell company Cellaviva AB, active in private family saving of stem cells from the umbilical cord. Stem cells can be expected to be used in Personalized Regenerative Medicine (PRM), for example for restoration of beta cell mass in diabetes patients where the autoimmune component of the disease has been arrested by ABT.
Diamyd Medical’s B-share is traded on Nasdaq Stockholm First North under the ticker DMYD B. Remium Nordic AB is the Company’s Certified Adviser.
For further information, please contact:
Anders Essen-Möller, President and CEO
Phone: +46 70 55 10 679. E-mail: anders.essen-moller@diamyd.com
Diamyd Medical AB (publ)
Kungsgatan 29, SE-111 56 Stockholm, Sweden. Phone: +46 8 661 00 26, Fax: +46 8 661 63 68
E-mail: info@diamyd.com. Reg. no.: 556242-3797. Website: www.diamyd.com.
Tags:

