FG001 is proven safe in patients undergoing surgery for cancer - a significant milestone for FluoGuide to further advance clinical development in aggressive brain cancer and in a prevalent indication
Copenhagen, Denmark, 8 October 2021 – FluoGuide A/S (“FluoGuide” or the “Company”) is pleased to announce that FG001 has been concluded to be safe and well tolerated by the dose escalation committee monitoring the ongoing phase I/II clinical trial. Safety data from 27 patients with aggressive brain cancer (including the just-completed seventh cohort) have been reviewed, and FG001 has proven to have a very satisfactory safety and tolerability profile in all 27 patients. In the completed seventh cohort, light was detected in all three patients
It is an important milestone for FluoGuide to now be able to demonstrate that FG001 is safe and well tolerated in patients undergoing cancer surgery at doses providing contrast in tumors. The safety and efficacy of FG001 is the starting point for advancing the development of FG001 towards both registration for guiding surgery of aggressive brain cancer as well as expanding its use into other cancer indications, e.g., more prevalent cancer forms such as lung cancer and breast cancer.
“The results have been promising from the start of the clinical trial. However, this is truly an important milestone for FG001 and for FluoGuide. It is now possible for us to advance FG001 towards approval for surgery of aggressive brain cancer, and in addition expand the clinical development to more prevalent indications such as lung or breast cancer – both around 30 times more prevalent than brain cancer”, says Morten Albrechtsen, CEO of FluoGuide A/S.
The dose escalation committee has reviewed the accumulated safety data from all 27 patients enrolled in the ongoing phase I/II clinical trial, including the dose to be carried forward into phase II of the ongoing phase I/II trial. Additional patients will be enrolled to determine the optimal time of administration of FG001 prior to surgery, leading to a planned initiation of phase II in Q1 2022. Finalization of the phase I/II trial is needed prior to initiation of the pivotal phase III trial required for marketing authorization of FG001 in aggressive brain cancer.
In the completed seventh cohort, light was detected in all three patients as expected and this further supports the establishment of efficacy for FG001. The collection of efficacy data is a stepwise process, where FG001 now has demonstrated both that the cancer lights up and that the contrast in the tumors is perceived as valuable by the neurosurgeons. The next steps are determining the histology of relevant dose from phase I (expected in Q1-22 following dose selection) and then the final efficacy result of phase II of the ongoing phase I/II trial in patients with aggressive brain cancer undergoing surgery (expected mid 2022).
The table below is a summary of the data from phase 1 of the clinical trial.
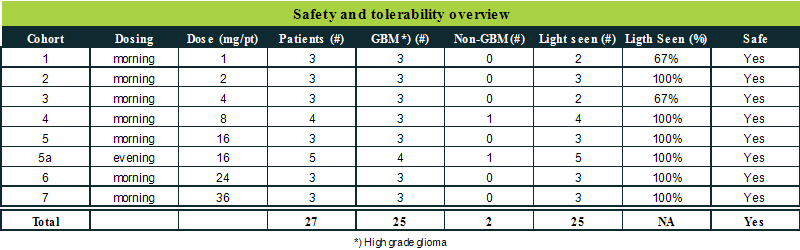
In total 27 patients have been included in the phase I part of the trial, of which 25 had aggressive brain cancer (high grade glioma) and 2 patients had cancer located in the brain of a different type than high grade glioma. FG001 has showed to have a very satisfactory safety and tolerability profile in the included 27 patients. In general, an increasing light intensity was observed with increasing dose levels.
“Several of the patients included in this trial were severely ill and the fact that FG001 has demonstrated an excellent safety profile in these patients supports a very broad use of FG001. I am also enthusiastic about the feedback we have received from the participating neurosurgeons as well as the conclusions made by the dose escalation committee.” says Andreas Kjær, founder and CMO of FluoGuide A/S.
FluoGuide will host an investor call on Monday 11 September 2021, at 9.30 am CEST. Morten Albrechtsen, CEO and Professor Andreas Kjær, CMO, will provide an update on the status for FG001 including safety and efficacy findings, as well as the plans and future milestones. To join the call please follow the link https://ir.live/fluoguide or Dial-in Number +1 (312) 248-9348, Dial-in ID Number: 953695#, Dial-in Passcode: 5170#
This disclosure contains information that Fluoguide is obliged to make public pursuant to the EU Market Abuse Regulation (EU nr 596/2014). The information was submitted for publication, through the agency of the contact person, on 08-10-2021 15:58 CET.
For further information, please contact:
Morten Albrechtsen, CEO
FluoGuide A/S
+45 24 25 62 66
ma@fluoguide.com
Certified Adviser:
Svensk Kapitalmarknadsgransking AB
Phone: +46 70 755 95 51
E-mail: ca@skmg.se
About FluoGuide
FluoGuide’s primary focus is to maximize surgical outcomes in oncology. The Company’s lead product, FG001, is designed to improve surgical precision by illuminating cancer cells intraoperatively. The improved precision enabled by FluoGuide’s products has a dual benefit – it reduces both the frequency of local recurrence post-surgery and lessens surgical sequelae. Ultimately, the improved precision will improve a patient’s chance of achieving a complete cure and will lower system-wide healthcare costs. The Company is conducting a proof-of-concept clinical study (phase I/II) to demonstrate the effect of FG001 in patients with high grade glioma. FluoGuide is listed on Nasdaq First North Sweden under the ticker “FLUO”.
About high grade glioma and glioblastoma
The first indication for FG001 is glioblastoma but FG001 has potential in several indications. Almost all patients with glioblastoma have a cancer expressing uPAR. A total of 60,000 patients gets high grade glioma and more than 30.000 patients are diagnosed with glioblastoma annually in the EU and US. Approximately 8-12 % of the patients are children. The prognosis for individuals with glioblastoma is very poor. Approximately 50 % of the patients die within 14 months and only 5 % are alive after five years from diagnosis. Precise removal of glioblastoma tumors is very difficult due the brain contains vital structures often near the cancer. Local reoccurrence of glioblastoma is common and happens in almost 100% of all patients.
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 954904.

