Nobles Medical Technologies II Receives Allowance Of Its Groundbreaking Patent For Next-Generation Suturing Device
NobleStitch™ QP Provides a Running Suture—
A Key Element To Close Septal Defects
Nobles Medical Technologies II (NMT2) today announced that it has received notice of allowance of its claims for its next-generation suturing technology. With the issuance of this patent, NMT2 will expand its suture technologies to include the ability to remotely perform a running suture. This technology will be applied to NMT2’s ASD-closure project currently under development.
Prof. Anthony Nobles, CEO of NMT2 and co-inventor on the allowed patent claims, commented, “This technology, which is currently under the working title NobleStitch™ QP, is completely different than our previous interrupted-suturing technology. The QP will allow us to place surgical patches and perform running sutures to close Atrial Septal Defects (ASD). ASD and Trans-Septal punctures are a priority for us at NMT2 as we have grown our international PFO business.”
Ben Brosch, President of NMT2 and co-inventor on the allowed patent claims, commented, “This is another example of our commitment to intellectual property, as well as our vision to keep expanding the resources of NMT2 to provide solutions for both PFO and ASD closure. It also comes just after our recent success of First-In-Man of a NobleStitch ASD closure, as well as Septal Aneurysm repair.”
Rich Babcock, a Director of NMT2, stated, “I continue to be amazed at the concentrated progress a small group of talented inventors can make in such a short period of time. In my many years of representing startups and entrepreneurs, I have never seen an inventor achieve as many strong patents as Prof. Nobles. NobleStitch™ QP continues to demonstrate his prowess in this field, and his essential contribution to NMT2 and its shareholders.”
About PFO Closure
A PFO is a relatively common heart defect characterized by an unsealed tunnel between the right and left atria of the heart. This defect has been known to be present in anywhere between 27%-38% of people. However, in a number of cases, it is benign.
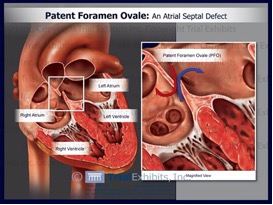
The PFO is formed as a trace of the fetal circulation. When the chambers of a human heart begin to develop, a tunnel is made between the right and left atria, allowing blood to flow directly from the venous circulation to the arterial circulation, circumventing the non-functioning fetal lungs. Following birth, the pressure differential between the right and left atria changes with newly operational blood flow to the fully functioning lungs. Because of this, the tunnel eventually closes completely within the first few months.
However, in some patients, the foramen ovale fails to seal and stays "patent". In patients with a Patent Foramen Ovale (PFO), the tunnel can reopen under elevated atrial pressure, such as coughing, or straining.
A key issue with PFO is that it gives a pathway for blood clots to pass directly to the arterial circulation without being filtered out by the capillary bed of the lungs. A PFO can also let deoxygenated blood and certain chemicals cross over to the arterial side. The presence of a PFO has been linked to a number of clinical issues, mainly stokes, migraines and chronic fatigue. Developments are being made to solidify the link between PFO and strokes or migraines, and to identify patients that would benefit from PFO closure.
For more information, please contact shareholder representatives:
Dru Dobbs
P. +1 714 427 6348
F. +1 714 427 6343
About Nobles Medical Technology II
Nobles Medical Technology II, Inc. was founded by Prof. Anthony Nobles with the intent of leveraging its technologies in the PFO, ASD-closure, and vascular-suturing marketplace. The company does business under the name of Nobles Medical II
(NMT II). Initial efforts of the company are focused on the innovative suture-based PFO closure system for closing the Patent Foramen Ovale (PFO), a tunnel between the right and left atria of the heart.
The NobleStitch™ is approved for PFO Closure and Cardiovascular suturing in the European Union. The NobleStitch™ EL is FDA cleared for vascular suturing in the United States. NobleStitch™ EL is distributed worldwide by HeartStitch®, Inc. (HeartStitch® is a registered trademark of HeartStitch, Inc.).
NobleStitch™ EL for PFO closure
Covered by or for use under U.S. and international patents including one or more of U.S. Patent Nos. 5860990, 6117144, 6245079, 6551331, 6562052, 6733509, 7004952, 7090686, 7803167, 8197497, 8197510, 8246636, 8348962, 8372089, 8469975, 8496676, 8709020, and 9131938.
For more on Nobles Medical Technologies II visit www.NoblesMedicalTechnology.com.
Tags:


