Integrum’s OPRA™ Implant System now offered at University of Southern California, Keck Medical Center of USC, California, USA
The program will offer comprehensive, highly specialized care for patients who have lost limbs from trauma or cancer.
Keck Medical Center of USC, one of the Top 20 hospitals nationwide as ranked by US News and World Report, will be offering osseointegration using Integrum’s OPRA™ Implant System, provided through its commercial partnership with Onkos Surgical. The medical center will be one of few in the country to offer this highly specialized, comprehensive care program for patients who have lost a limb due to trauma or cancer.
Approximately 2 million people in the US live with limb loss. Up to three-quarters of patients who undergo a lower-extremity amputation experience post-surgical issues in the prosthetic socket. Osseointegration fuses the prosthetic limb directly into the bone rather than in the socket. This improves control and mobility over the prosthetic limb, as well as reduces pain and the risk of complications with a socket. Integrum’s OPRA™ Implant System was approved by the US Food and Drug Administration (FDA) in 2015 for use in the US under a Humanitarian Use Device (HUD) designation, which was reviewed through the Humanitarian Device Exemption (HDE) pathway.
Keck Medical Center’s multidisciplinary osseointegration program will be led by Lawrence Menendez, MD, orthopaedic surgeon with Keck Medicine of USC. Dr. Menendez specializes in managing bone and soft tissue tumors and is experienced in performing complex limb-sparing and prosthetic management procedures.
“Our team is committed to providing patients with musculoskeletal conditions with cutting-edge treatments and personalized care,” says Dr. Menendez, who is also professor of clinical orthopaedic surgery at the Keck School of Medicine of USC.
“We are excited to work with this team-oriented patient program through Onkos Surgical,” says Maria Lopez, CEO of Integrum. “Keck Medicine has a history of providing innovative and personalized care, and the addition of this program follows their tradition of advanced medicine.”
To make an appointment with a Keck Medicine orthopaedic surgeon or learn more about its services, visit KeckMedicine.org or call (800) USC-CARE.
About the OPRA™ Implant System
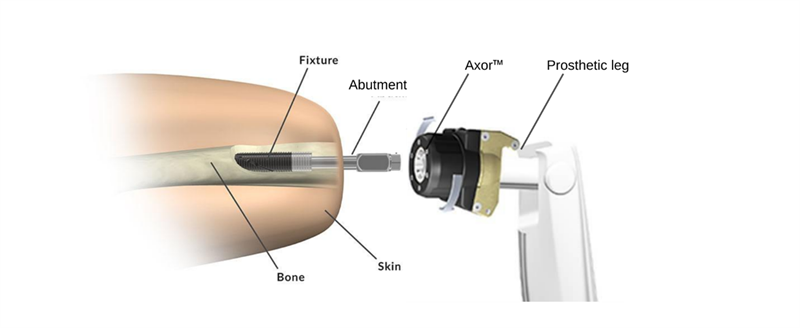
Integrum’s OPRA™ Implant System is the only FDA-approved, bone-anchored prosthetic solution available in the United States. OPRA™ Implant System consists of an anchorage element (Fixture) and a skin penetrating device (Abutment). The Fixture is surgically inserted into the femur, and after a healing time of several months, the Abutment is connected to the Fixture. The prosthetic leg is then attached directly to the Abutment via the Axor™, a prosthetic connection and safety device.
This disclosure contains information that Integrum AB is obliged to make public pursuant to the EU Market Abuse Regulation (EU nr 596/2014). The information was submitted for publication, through the agency of the contact person, on 12-10-2020 14:30 CET. Integrum is listed on Nasdaq First North in Stockholm. Erik Penser is the Company's Certified Adviser.
For further information, please contact:
Maria Lopez, CEO
Cell. 0708-46 10 69
Email: maria.lopez@integrum.se
Certified Adviser:
Erik Penser Bank AB
Tel. +46 8 463 8000
Email: certifiedadviser@penser.se
About Integrum
Integrum AB is a publicly traded company (INTEG B: Nasdaq First North exchange) based outside of Gothenburg, Sweden with a U.S. subsidiary in San Francisco, CA. Since 1990 the OPRA™ Implant System has been helping individuals with amputations towards an improved quality of life. Thorough surgical experience gained over more than three decades from 500 surgeries in 14 countries has led to the development of Integrum’s system for bone-anchored prosthetics – a beneficial alternative to the traditionally used socket prosthesis. Integrum’s OPRA™ Implant System was approved by the U.S. Food and Drug Administration (FDA) in 2015 for use in the U.S. under a Humanitarian Use Device (HUD) designation which was reviewed through the Humanitarian Device Exemption (HDE) pathway. The OPRA™ Implant System is the only FDA approved technology for bone anchored prosthetics available in the U.S. More information on the company and its innovative solutions for amputees can be found at www.integrum.se.
About Onkos Surgical
Based in Parsippany, N.J., Onkos Surgical is a privately held surgical oncology company founded in 2015. We believe that individuals with cancer requiring surgery deserve solutions designed specifically for them. This principle is the driving force behind our Precision Oncology initiatives. Built on a digital platform, our solutions are rooted in unmatched expertise in patient imaging analysis, personalized surgical planning, and the latest advancements in 3D printing. At Onkos, we are passionate about reducing complexity for our customers and addressing the clinical challenges associated with tumor surgery. www.onkossurgical.com


