New study shows Integrum’s OPRA™ Implant System to be safe and durable method to treat thumb amputees
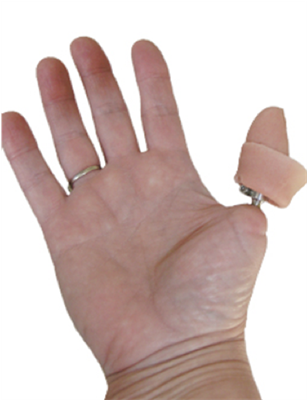
Integrum, a leading provider of innovative solutions for bone anchored prosthetics, is pleased to announce that the Journal of the American Academy of Orthopaedic Surgeons (JAAOS) has published details of a study featuring Integrum’s OPRA™ implant system to enable osseointegrated thumb reconstruction. The study results, published in the January 2019 issue, indicate that the OPRA™ Implant System is a safe and durable method to treat patients with thumb amputations.
The retrospective single center study, based on preoperative and postoperative data collected from 13 thumb amputees, concludes that the “treatment of thumb amputees using bone-anchored percutaneous prostheses with the osseointegration technique seems to be a safe, durable method with excellent short- and medium-long follow-up results. Using the latest design (after 2005), there is a 100% cumulative success rate, up to 10 years of follow-up. Although the patient population is limited, the osseointegrated reconstruction of thumbs is shown to offer a valuable psychological, functional, and rehabilitative potential in daily life activities for patients.”
Additional information
Study reference: Thumb Amputations Treated With Osseointegrated Percutaneous Prostheses With Up to 25 Years of Follow-up; Li, Yan, MD, PhD; Kulbacka-Ortiz, Katarzyna, MS; Caine-Winterberger, Kerstin, MOT; Brånemark, Rickard, MD, PhD; JAAOS Global Research & Reviews: January 2019 - Volume 3 - Issue 1 - p e097; doi: 10.5435/JAAOSGlobal-D-18-00097
Link to study: https://bit.ly/2S1zqKF
About journal: The Journal of the American Academy of Orthopaedic Surgeons (JAAOS), an official journal of the American Academy of Orthopaedic Surgeons, is a peer-reviewed, clinical review publication for scientists, researchers and clinicians interested in the clinical diagnosis and management of musculoskeletal conditions, as well as the current state of orthopaedic practice.
For more information, please contact:
Rutger Barrdahl, CEO
Tel. +46 705 23 39 55
rutger.barrdahl@integrum.se
www.integrum.se
Certified Adviser:
Erik Penser Bank AB
Tel. +46 8 463 8000
certifiedadviser@penser.se
About Integrum
Integrum AB is a publicly traded company (INTEG B: Nasdaq First North exchange) based outside of Gothenburg, Sweden, with a U.S. subsidiary in San Francisco, CA. Since 1990 osseointegration, the science behind the OPRA™ Implant System, has been helping individuals with amputations towards an improved quality of life. Surgical experience gained over almost three decades from 500 surgeries in 13 countries has led to the development of Integrum’s system for bone-anchored prosthetics – a beneficial alternative to the traditionally used socket prosthesis. Integrum’s OPRA™ Implant System was approved by the U.S. Food and Drug Administration (FDA) in 2015 for use in the U.S. under a Humanitarian Use Device (HUD) designation which was reviewed through the Humanitarian Device Exemption (HDE) pathway. The OPRA™ Implant System is the only FDA approved technology for above knee, bone anchored prosthetics available in the U.S. More information on the company and its innovative solutions for amputees can be found at www.integrum.se.
Tags:


