IRLAB nominates two new candidate drugs and updates the pipeline
IRLAB nominates two new drug candidates – IRL942 and IRL1009 – in the P001 research program, where development work is initiated for IRL942 and IRL1009 as a backup. Further, a new preclinical discovery program, P003, is introduced in the pipeline. The P003 program aims at developing a new drug that can replace levodopa as standard medication in early Parkinson's disease. The project is in discovery phase. Based on the promising results from the completed Phase I and Phase IIa programs with IRL752, IRLAB is now planning a forthcoming Phase II study with the primary goal to study efficacy in Parkinson’s disease patients with dementia.
"The nomination of the two new candidate drugs and the update clarifies that IRLAB focuses on IRL790 and IRL752 and utilizes our competitive advantage following the positive results in initial clinical trials. The new drug candidates are intended to complement the IRL790 and IRL752 projects with a strong commitment in Parkinson’s disease", said Nicholas Waters, CEO of IRLAB Therapeutics.
"It is very rewarding that we, as a young company, have the ability to strengthen our pipeline with a new research program while nominating two more promising drug candidates in one of our ongoing projects. Our research organization has once again demonstrated the efficiency of the ISP platform", said Clas Sonesson, Chief Scientific Officer (CSO) of IRLAB.
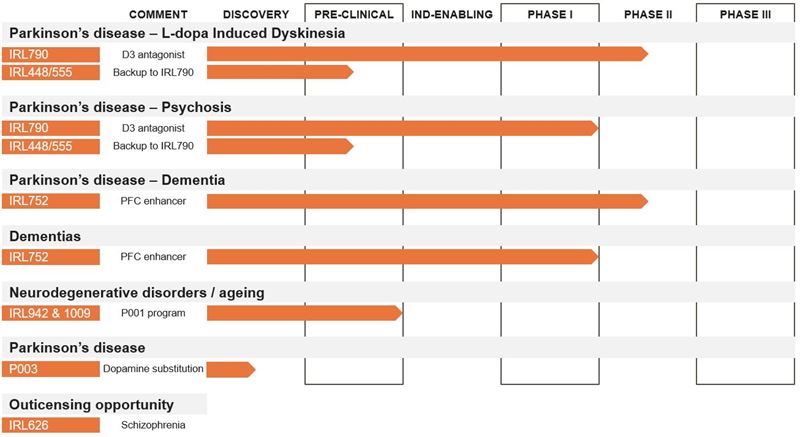
Figure: IRLAB's pipeline
P001
IRLAB's Pharmaceutical Project P001 focuses on the treatment of inadequate executive functions that lead to complications in movement disorders as well as mental and cognitive decline. In the project, two drug candidates have now been nominated, IRL942 and IRL1009, where development work is initiated for IRL942 with IRL1009 as backup compound. IRL942 and IRL1009 have been discovered with IRLAB's unique research platform, ISP.
Both IRL942 and IRL1009 address the relationship between motor and cognitive functions. The new drug candidates are intended to offer better effect and fewer side effects than today's treatment options and will initially be developed for non-motor symptoms in neurodegenerative diseases and aging.
IRL942 has been selected as the project's leading substance after extensive preclinical studies, in vivo and in vitro, including efficacy studies, gene expression, pharmacokinetics and safety. IRL1009 has similar effects and the compound will be developed in series with IRL942. The drug candidate IRL942 will, as a first step, be taken through a preclinical development program to meet the regulatory requirements for obtaining permission to conduct clinical Phase I studies.
IRLAB has received a notice of allowance from the U.S. Patent Office (USPTO) regarding patent applications relating to IRL942 and IRL1009, which means that IRLAB will be granted a U.S. patent. The patent is a composition of matter patent.
The development of the preclinical assets does not entail any additional funding needs.
P003
Since the 1960s, levodopa, in its various formulations, has been the standard treatment for Parkinson's disease. The treatment is generally effective but lacks efficacy in certain symptom domains and has, especially after prolonged treatment, side effects.
With the help of the ISP research platform, IRLAB has developed a group of molecules with the potential to be developed into treatments that could substitute levodopa as treatment in newly diagnosed Parkinson's disease.
IRL790
After a slightly reduced recruitment pace in August, IRLAB and the company's CRO, The Clinical Trial Consultants (TCTC), estimate that the recruitment target of 74 patients will be reached in the fourth quarter of 2018, which is a deviation from the assessment given in January 2018. Topline results are presented immediately after completion of treatment and data analysis.
A total of 16 UK clinics are involved in the placebo-controlled, double-blind and randomized Phase IIa study (IRL790C003), which has as its primary goal to study the effects of IRL790 on levodopa induced dyskinesias (PD-LIDs). Leading clinics focused on Parkinson's disease began recruitment in the study in April 2018. Participating physicians and hospitals are highly committed to the study.
Work to further strengthen the intellectual property protection of the drug candidate IRL790 continues. IRL448 and IRL555 are compounds developed in parallel with IRL790. The compounds have properties that reinforce the IRL790 project and the protection of the indications IRL790 addresses. The patents around IRL448 and IRL555 are now added to the IRL790 program.
IRL752
Based on the promising results from the completed Phase I and Phase IIa programs with IRL752, the company is currently planning a Phase II study with the primary goal of studying effects on axial motor functions and cognitive functions.
IRL626
IRLAB focuses on Parkinson's disease and the clinical projects around the IRL790 and IRL752. IRL626 is indicated in schizophrenia and the project is taken out of the company's pipeline and is kept available for outlicensing to partners with development strategies in schizophrenia.
For further information
Nicholas Waters, CEO
Phone: +46 730 75 77 01
E-mail: nicholas.waters@irlab.se
Clas Sonesson, CSO
Phone: +46 730 75 77 00
E-mail: clas.sonesson@irlab.se
This information is information that IRLAB Therapeutics AB (publ) is obliged to make public pursuant to the EU Market Abuse Regulation. The information was submitted for publication, through the agency of the contact person set out above, at 08:59 CET on September 19, 2018.
FNCA Sweden AB is the company's Certified Adviser on Nasdaq First North Premier.
About IRLAB
IRLAB is a research and development company, listed on Nasdaq First North Premier, focused on development of novel therapies for the treatment of neurodegenerative diseases, in particular Parkinson’s disease.
IRLAB has two clinical candidate drugs, IRL752 and IRL790, focused on medical needs in Parkinson’s disease. IRLAB also has additional programs in pre-clinical stages.
IRLAB’s research is aimed at discovery and development of new candidate drugs addressing unmet medical need in diseases of the central nervous system, using the unique and proprietary integrative screening process, ISP.
IRLAB is based in Gothenburg, Sweden. The operations are mainly carried out through the subsidiary Integrative Research Laboratories Sweden AB.
For more information, please visit www.irlab.se.


