NCCN Unveils Evidence Blocks for CML and Multiple Myeloma
New visual tool illustrates five dimensions of value within NCCN Guidelines
SAN FRANCISCO, Calif., (October 16, 2105) — The National Comprehensive Cancer Network® (NCCN®), a not-for-profit alliance of 26 of the world's leading cancer centers, today unveiled its new value initiative—the NCCN Evidence Blocks™, published within new versions of the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Myelogenous Leukemia (CML) and Multiple Myeloma.
“In cancer care, the most important value perspective is that of the individual patient,” said Robert W. Carlson, MD, chief executive officer, NCCN. “NCCN Evidence Blocks will educate providers and patients about the efficacy, safety, and affordability of systemic therapy, serving as a starting point for shared decision-making based on the individual patient’s value system.”
The announcement was made at the NCCN 10th Annual Congress: Hematologic Malignancies™ at the San Francisco Marriott Marquis. The NCCN Annual Congress focuses on the increasingly complex treatment of hematologic malignancies and new approaches that have been incorporated into patient management, including the use of drugs, biologics, and diagnostics.
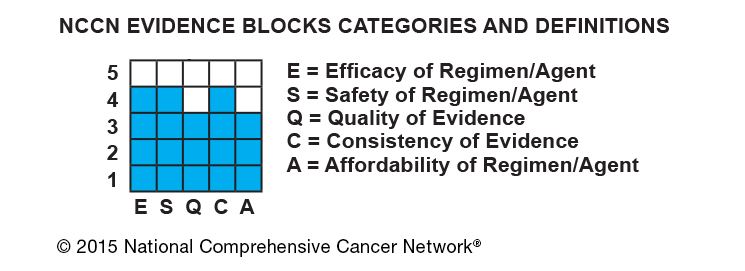
The NCCN Evidence Blocks™ are published in a new version of the NCCN Guidelines and are intended as a visual representation of five key value measures that provide important information about specific Guidelines recommendations:
- Efficacy of regimens,
- Safety of regimens,
- Quality and quantity of evidence for regimens,
- Consistency of evidence for regimens, and
- Affordability of regimens.
In a rapidly evolving field like oncology, thousands of new publications are released each year, adding to the existing body of knowledge and resulting in improvement in outcomes. In publishing the NCCN Guidelines, panel members are able to integrate new findings with existing information to determine what the evolving standard of care should be for a given disease state. Implicit in the evaluation of each treatment is the efficacy and expected associated toxicities, as well as the quality, quantity, and consistency of the evidence supporting the recommendation.
By adding affordability to NCCN’s existing criteria for evaluating treatment options, patients will be empowered to identify, alongside their physician, optimal treatment based on clinical and economic considerations that are of most value to them. The affordability measurement represents an estimate of overall total cost of a therapy, including but not limited to acquisition, administration, in-patient vs. out-patient care, supportive care, infusions, toxicity monitoring, antiemetics and growth factors, and hospitalization.
“Some patients will want an emerging therapy even with limited data; others will be most concerned about the expected side effects of the treatment indicated in the safety column. Still others may be very sensitive to cost,” Dr. Carlson said. “By considering the attributes of the range of possible therapies, the health care provider and the patient can discuss the benefits and drawbacks of each option and come to a decision most acceptable to the individual.”
By the end of 2015, NCCN expects to publish NCCN Evidence Blocks™ for systemic therapies (not surgery or radiation therapy) in the NCCN Guidelines for Breast, Colon, Non-Small Cell Lung, and Rectal Cancers; NCCN Evidence Blocks™ for systemic therapies are expected to be contained within the complete library of NCCN Guidelines by the end of 2016.
In addition to the usefulness of the conversation that the affordability dimension produces, the design of the resulting NCCN Evidence Blocks™, with its signature blue squares, provides oncologists a valuable reference.
“In an age of visual information, the NCCN Evidence Blocks™ are a time-saving tool for efficient scanning and interpretation of multiple therapy options in an efficient format,” said Dr. Carlson.
The concept of NCCN Evidence Blocks™ was first announced in March 2015 during the NCCN 20th Annual Conference: Advancing the Standard of Cancer Care™ in Hollywood, Florida.
The NCCN Guidelines are the recognized standard for clinical policy in cancer care and are the most thorough and most frequently updated clinical practice guidelines available in any area of medicine. Available free of charge to registered users of NCCN.org, the NCCN Guidelines cover 97 percent of all patients with cancer.
In the near term, NCCN will continue to publish two sets of NCCN Guidelines: those including NCCN Evidence Blocks™ and those published without. The NCCN Evidence Blocks™ are not currently published in the NCCN Guidelines for Patients®, and are intended for use in the United States only.
For more information about NCCN Evidence Blocks™, visit NCCN.org/EvidenceBlocks and follow NCCN on Twitter: @NCCNNews, #NCCNValueChat.
###
About the National Comprehensive Cancer Network
The National Comprehensive Cancer Network® (NCCN®), a not-for-profit alliance of 26 of the world's leading cancer centers, is dedicated to improving the quality and effectiveness of care provided to patients with cancer. Through the leadership and expertise of clinical professionals at NCCN Member Institutions, NCCN develops resources that present valuable information to the numerous stakeholders in the health care delivery system. As the arbiter of high-quality cancer care, NCCN promotes the importance of continuous quality improvement and recognizes the significance of creating clinical practice guidelines appropriate for use by patients, clinicians, and other health care decision-makers. The primary goal of all NCCN initiatives is to improve the quality, effectiveness, and efficiency of oncology practice so patients can live better lives.
The NCCN Member Institutions are: Fred & Pamela Buffett Cancer Center, Omaha, NE; Case Comprehensive Cancer Center/University Hospitals Seidman Cancer Center and Cleveland Clinic Taussig Cancer Institute, Cleveland, OH; City of Hope Comprehensive Cancer Center, Los Angeles, CA; Dana-Farber/Brigham and Women’s Cancer Center | Massachusetts General Hospital Cancer Center, Boston, MA; Duke Cancer Institute, Durham, NC; Fox Chase Cancer Center, Philadelphia, PA; Huntsman Cancer Institute at the University of Utah, Salt Lake City, UT; Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance, Seattle, WA; The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD; Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, IL; Mayo Clinic Cancer Center, Phoenix/Scottsdale, AZ, Jacksonville, FL, and Rochester, MN; Memorial Sloan Kettering Cancer Center, New York, NY; Moffitt Cancer Center, Tampa, FL; The Ohio State University Comprehensive Cancer Center - James Cancer Hospital and Solove Research Institute, Columbus, OH; Roswell Park Cancer Institute, Buffalo, NY; Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO; St. Jude Children’s Research Hospital/The University of Tennessee Health Science Center, Memphis, TN; Stanford Cancer Institute, Stanford, CA; University of Alabama at Birmingham Comprehensive Cancer Center, Birmingham, AL; UC San Diego Moores Cancer Center, La Jolla, CA; UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, CA; University of Colorado Cancer Center, Aurora, CO; University of Michigan Comprehensive Cancer Center, Ann Arbor, MI; The University of Texas MD Anderson Cancer Center, Houston, TX; Vanderbilt-Ingram Cancer Center, Nashville, TN; and Yale Cancer Center/Smilow Cancer Hospital, New Haven, CT.
Clinicians, visit NCCN.org. Patients and caregivers, visit NCCN.org/patients.
Katie Kiley Brown, NCCN
215.690.0238 (o); 610.573.3893 (c)
Tags:


