PHI establish US subsidiary to foster US partnerships
As part of the ongoing expansion of activities on the US market, the Board of Directors of Phase Holographic Imaging (PHI) has initiated the process of establishing a fully owned subsidiary in the US.
In February PHI signed a technology assessment agreement with a major US-based supplier of antibodies for cancer immunotherapy use. A joint seminar recently made it clear how exceptionally well the companies’ products complement each other. Together the respective product lines offer a complete solution to both activate immune cells and to study the effect of the activation by quantifying the immune cells ability to attack and destroy tumor cells. The encouraging evaluation results will form the basis for the continued discussions and the future relationship between the companies.
Cancer immunotherapy, also known as immuno-oncology, is a form of cancer treatment that uses the body’s own immune system to prevent or treat cancer. The most successful and promising cancer immunotherapy treatments use proteins in the form of antibodies, to activate the patient’s immune cells to attack and destroy tumor cells. As a result of the clinical successes and its potential to cure all cancer forms, the cancer immunotherapy field has experienced dramatic growth during the past few years. In September 2018, there were 2 250 clinical trials aiming to further improve antibody-based immunotherapy, an increase of 748 trials (33%) since the previous year. A staggering 380 000 patient volunteers will be needed to complete all current trials.
Against this background and to further foster several future US partnerships, PHI’s Board of Directors has initiated the establishment of a fully owned subsidiary in Boston Mass. USA — Phase Holographic Imaging PHI Inc.
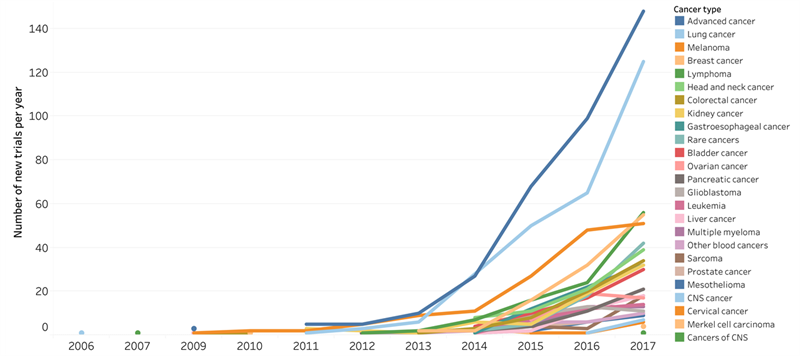
The evolution of the most common cancer immunotherapy approach (PD-1/L1 inhibitors) between 2006 and 2017. 1 768 clinical trials were started between 2006 and 2017. Data for 2018 was excluded to avoid an artificial decrease, as the cut-off date was September 2018. Source: Cancer Research Institute.
References
New Report Charts Dramatic Growth in the Global Clinical Trial Landscape for PD-1/L1 Immune Checkpoint Inhibitors, Cancer Research Institute (2018)
For additional information, please contact:
Carolina Silvandersson
E-mail: ir@phiab.se
Web: www.phiab.se
Phase Holographic Imaging (PHI) leads the ground-breaking development of time-lapse cytometry instrumentation and software. With the first instrument introduced in 2011, the company today offers a range of products for long-term quantitative analysis of living cell dynamics that circumvent the drawbacks of traditional methods requiring toxic stains. Headquartered in Lund, Sweden, PHI trades through a network of international distributors. Committed to promoting the science and practice of time-lapse cytometry, PHI is actively expanding its customer base and scientific collaborations in cancer research, inflammatory and autoimmune diseases, stem cell biology, gene therapy, regenerative medicine and toxicological studies.


