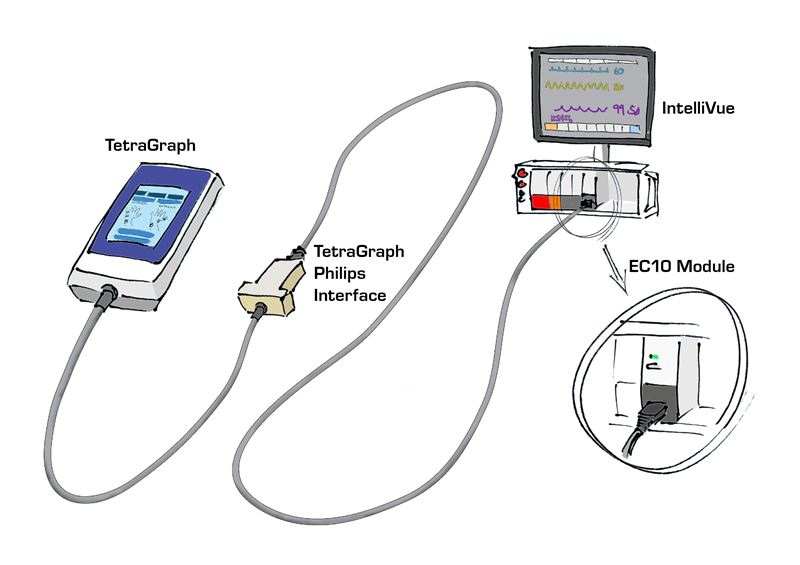
Launch of TetraGraph Philips Interface
Press release: Uppsala, December 3, 2019. As previously communicated in a Press release 2018, Senzime AB (publ) signed a license and cooperation agreement with Philips to allow the TetraGraph Neuromuscular Monitoring System to communicate with Philips IntelliVue patient monitors worldwide. The TetraGraph Philips Interface is now ready for launch.
Philips is one of the global leaders in the field of patient monitoring. The centralized Philip IntelliVue systems is one of the most advanced technologies in the field. Flexible access to critical physiologic patient data provides advanced clinical decision support, enhancing care and streamlining workflow.
Senzime’s TetraGraph neuromuscular monitor is a unique digital system designed to address the needs of perioperative monitoring of physiologic data in surgical patients receiving general anesthesia and muscle relaxation using neuromuscular blocking drugs (NMBAs). The TetraGraph stimulates a peripheral nerve and measures, analyzes and displays in real-time the muscle function in surgical patients who receive NMBAs as part of their general anesthetic.
Interconnected medical devices are valuable resources in the delivery of patient care. This launch enables TetraGraph data to be transmitted via a cable to Philips IntelliBridge EC10 compatible IntelliVue MP/MX series Patient Monitors and to Patient Information Center IX through IntelliBridge EC40/80 in order to be displayed together with other critical patient data.
Pia Renaudin, CEO of Senzime states, "With our interface critical patient information provided by the TetraGraph can be displayed on a wide range of Philips IntelliVue patient monitors worldwide. Our data will be treated no differently than any other data in the system, thus enabling the user to integrate it in their standard Patient Data Management System.”
This interface is in line with Senzimes corporate strategy seeking alliances with the mayor providers of large central monitoring stations. The integration is conditioned upon local regulatory approvals of TetraGraph.
For further information, please contact:
Pia Renaudin, CEO of Senzime AB
Tel: +46 (0)70-813 34 17, email: pia.renaudin@senzime.com
TO THE EDITORS
About Senzime
Senzime develops and markets patient monitoring systems, driven by unique algorithms and sensors. The company's system is called TetraGraph, a medical technology device that digitally and continuously measures the degree of neuromuscular blockade in the patient. The goal is improved clinical precision and simplified management in healthcare. By preventing complications and enabling healthcare professionals to follow guidelines and drug recommendations, TetraGraph can contribute to shorten hospital stays and lower healthcare costs. The vision is a world without anesthesia related complications, where everyone wakes up safely after surgery. Senzime operates in growing markets that in Europe and the United States are valued in excess of SEK 10 billion. The company's shares are listed on Nasdaq
First North Growth Market (ticker SEZI). FNCA Sweden AB, +46 (0)8-528 00 399, info@fnca.se is Certified Adviser for Senzime. www.senzime.com
Senzime AB
Ulls väg 29B
756 51 Uppsala, Sweden
Phone : + 46 18 51 56 40
Tags:


