Senzime files new FDA application for TetraGraph
Press release: Uppsala, March 28, 2019. Senzime AB (publ) today announces the filing of the new 510(k) application to the US Food and Drug Administration (FDA) for the TetraGraph system. This filing is in line with previous communication and strategy to decrease the overall time to reach FDA clearance and subsequent introduction on the US market.
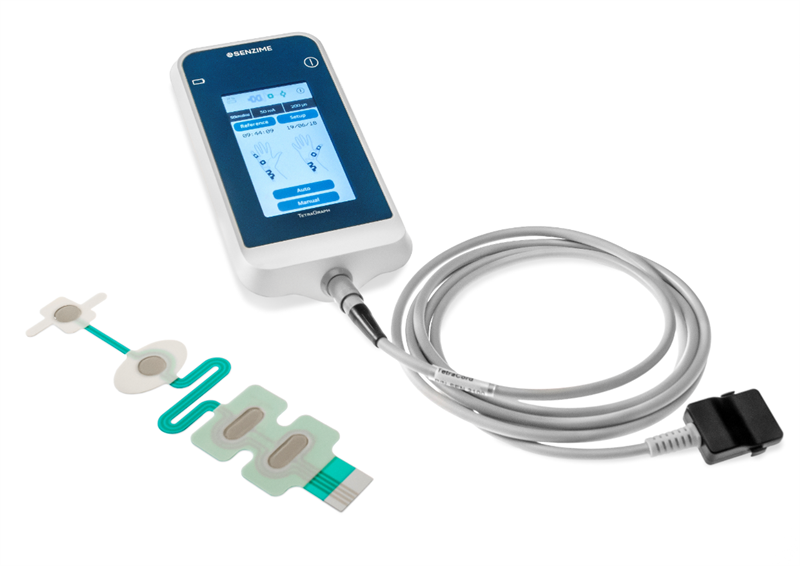
The FDA application is part of Senzime's strategic launch plan for the TetraGraph system, with primary focus of launching in Europe, Japan, Korea and the United States – central markets for monitoring patients undergoing surgery with general anesthesia and muscle relaxants. The majority of 510(k) applications are cleared within six months.
For further information, please contact:
Pia Renaudin, CEO of Senzime AB
Tel: +46 (0)70-813 34 17, email: pia.renaudin@senzime.com
TO THE EDITORS
About Senzime
Senzime develops unique patient-oriented monitoring systems that make it possible to assess patients' biochemical and physiological processes before, during and after surgery. The portfolio of technologies includes bedside systems that enable automated and continuous monitoring of life-critical substances such as glucose and lactate in both blood and tissues, as well as systems to monitor patients’ neuromuscular function perioperatively and in the intensive care medicine setting. The solutions are designed to ensure maximum patient benefit, reduce complications associated with surgery and anesthesia, and decrease health care costs. Senzime operates in growing markets that in Europe and the United States are valued in excess of SEK 10 billion. The company's shares are listed on Nasdaq First North (ticker SEZI). FNCA Sweden AB, +46 (0)8-528 00 399, info@fnca.se, is Certified Adviser for Senzime. www.senzime.com
This information is insider information that Senzime AB is obliged to make public pursuant to the EU Market Abuse Regulation. The information was submitted for publication through the agency of the contact person set out above, on March 28th, 2019 08:45
Tags:


