Results from clinical study for patient 9 and 10
In SpectraCure’s Phase 1 study for treatment of patients with recurrent prostate cancer, preliminary results have been reported for the most recently treated patients. The treatment method, called photodynamic therapy (PDT), means that the patient is given a light-activated drug that accumulates in the tumour. When the cancerous tissue is illuminated using SpectraCure’s IDOSE technique, the drug is activated, and the tumour eliminated.
The treatment effect is evaluated in the study with magnetic resonance imaging (MR) one week after the treatment. The evaluation of the MR images for the most recently treated patients shows good effect, which is consistent with previous results at the corresponding dose level. The preliminary results also show that the effect in the treated area corresponds well with the treatment plan of SpectraCure’s IDOSE system.
- The results are promising, says Johannes Swartling, CTO. Preliminarily, we can draw two important conclusions. First, we see a clear dose response, meaning that the treatment effect depends on the dose in a predictable way. Second, the treatment plan in IDOSE provides good precision.
- We see a good treatment effect and tissue destruction in the tumour area, comments Dr. Nathan Perlis, the surgeon who conducted the treatments at the Princess Margaret Cancer Centre in Toronto.
It should be emphasized that the main objective of the Phase 1 study is to demonstrate that SpectraCure’s treatment method is safe, and to establish the correct dose level. The preliminary results also imply that the method has the intended effect, which is a secondary goal for the phase 1 study.
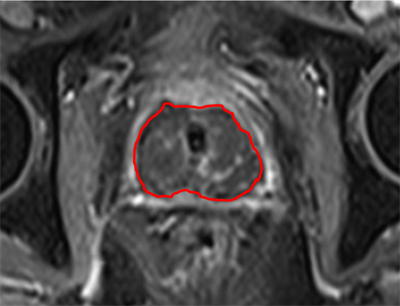
MR images
Image 1 shows the effect of the entire prostate gland treatment of patient 9. The dark area indicates good treatment effect.
Image 2 shows the effect of the treatment of patient 10. Treatment for patient 10 was focal, meaning that the purpose was only to treat the part where the tumour was present and not the entire prostate gland. In the image this is shown as the dark area in the lower part of the prostate. The light part of the prostate, in the upper part of the image, indicates untreated tissue.
For further information, please contact:
SpectraCure AB publ, CEO, Masoud Khayyami, phone: +46 (0) 70 815 21 90
Certified Adviser is G&W Fondkommission, phone: +46(0) 8 503 000 50
This information is information that SpectraCure AB is required to disclose under the EU Market Abuse Regulation. The information was provided, through the contact of the above contact person, for publication on January 28th, 2019.
SpectraCure in short
SpectraCure was founded in 2003 as a spin off from Lund University departments for medical laser applications and physics. The company focuses on cancer treatments using medical systems with laser light sources and reactive drugs, which is referred to as "Interstitial Photodynamic Therapy", PDT, a treatment methodology suitable for internal solid tumours of various kind, e.g. prostate and abdominal salivary glands, but also other indications such as cancer tumours in the head and neck region
Tags:



