Transdermal Specialties to Unveil Breakthrough Insulin Patch Technology at the American Diabetes Associations 72nd Scientific Session
Patented, Insulin Patch Offers Needle Free Disease Management
TSI’s New “Set IT And Forget IT” Insulin Delivery System Offers Totally Non-Invasive Insulin Delivery For Both Type-1 and Type-2 Diabetic Patients.
Broomall, PA – May 30, 2012 - Transdermal Specialties Inc., a pioneer in painless, injection-free ultrasonic transdermal drug-delivery systems for the pharmaceutical industry, will unveil its breakthrough Insulin Patch Technology at the American Diabetes Association's 72nd Annual Scientific Meeting, June 9 -11, 2012 in Philadelphia.
Cutting Edge Technology – The U-Strip is an active transdermal delivery system using a patented alternating ultrasonic waveform process to enlarge the diameter of the skin pores, enabling large molecule drugs to permeate through the skin (Stratum Corneum) into the Dermis through the sweat pores and then into the blood stream - All Non-invasively.

Key Advantages
- Delivers Insulin for Both Basal and Bolus Needs.
- Patches Available In Four Different Doses, 25, 50, 100 And 150 Units.
- Electronic Delivery System Tracks Dosing History and Glucose Readings and Downloads Data to Physician for Progress Monitoring
How it Works - The Special Ultrasound System expands the skin pores to enable large molecule drugs like insulin to be passed transdermally into the skin- No Needles. The U-Strip Technology expands the number of Drugs which could be delivered transdermally.
Confocal Imaging – The photograph of the skin below shows the before & after pore expansion effect of the ultrasound.
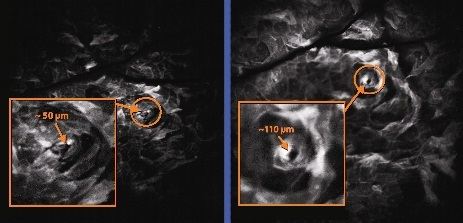
Standard Pore size Expanded via Alternating Ultrasound
The U-STRIP ™with its Programmable Transdermal Insulin Patch is
Totally Non-invasive and Comparable to an Insulin Pump

Extensive Clinical Program Underway - Transdermal Specialties is currently pursuing clinical trials of its U-Strip Insulin Patch. The Company’s goal is to complete the last two clinical trials needed for FDA approval in the next 18 months. 12 clinical trials have already been successfully completed, with over 125 diabetics screened so far. The results will be showcased at the American Diabetes Convention. The next planned clinical trials will involve over 500 diabetic patients in two trials before heading to the FDA. The HPT- 6 trial (summer 2012) will examine the tendency of the Insulin Patch to reach the same glucose level as a pump with less insulin. This implies a higher efficiency for the Patch. This trial will also compare the speed of delivery vs. Injection to determine if the Patch can be more effective in Morning Glucose Reduction for those patients waking with high glucose numbers. The HPT-7 trial (2013) will focus on a real-world study of 500 Type-2 diabetics, who will conduct an at-home study over 4 months to track their A1C levels.
Gateway to Improved Diabetes Care- Commented Bruce K. Redding, Jr., “The Company’s U-Strip system is being designed to blend Transdermal and Electronic control systems for improved diabetes care”. The Insulin Patch component offers a safe and painless alternative to injections with the promise of reduced side effects and improved insulin uptake efficiencies for the patient. The ultrasound actually reduces the quantity of insulin needed for effective glucose control and speeds the delivery over a pump or even direct injection. Improved patient monitoring and reporting of the Control Device enables better tracking of treatment programs and the new “Set-it and Forget-It” function means more regular glucose control during both evening and daytime hours. The U-Strip represents a major advance in diabetes care.”

“We believe the Transdermal Specialties’ platform technology has the potential to increase the number of pharmaceuticals which may be delivered transdermally from a number less than 20 currently expanding past the 175 formulations already modeled by Transdermal Specialties. The U-Strip technology expands the number of pharmaceuticals capable of transdermal delivery both for short term use and for solutions to the chronically ill” The Insulin and expanded transdermal profile pharmaceuticals currently represent sales of $24 billion. The transdermal version of these drugs, many of which are loosing patent protection in the next few years, offers a major Line Extension potential”.
About Transdermal Specialties, Inc – Transdermal Specialties, Inc, has been developing the U-Strip technology for drug delivery in general, with insulin delivery as the market focus, and a sister technology for improved skin care (the U-Wand) since 2004. The Company expects its patented ultrasonic delivery systems to be commercialized in late 2012 with the U-Wand Anti-Acne system and the earliest opportunity for Insulin Delivery in 2014.
Experimental Device. Not Yet Approved By the Food and Drug Administration of the USA
TO LEARN MORE ABOUT THIS EMERGING TECHNOLOGY: www.TransdermalSpecialties.com
Contact:
Theodore J. van de kamp
Phone Number: P:203-857-4887/M:203-984-1813
Email: tvandekamp@transdermalspecialties.com
Tags:






