Articular cartilage can heal partially after injury – researchers found a new mechanism
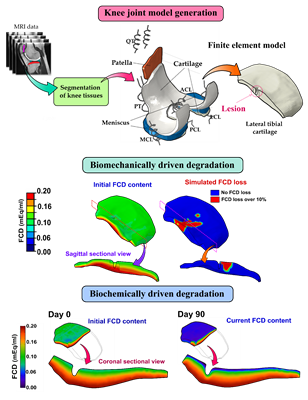
Post-traumatic osteoarthritis is a musculoskeletal disorder shadowing especially the life of young and active population. Inhibition of cartilage damage progression with early intervention is crucial. To predict how an intervention would affect the state of an injured knee joint, knowledge of biomechanical and inflammatory aspects of osteoarthritis progression is necessary. For the first time, researchers have now captured both mechanisms in a physics-based computational model predicting cartilage degradation and a possible recovery scenario after anterior cruciate ligament injury and reconstruction. The fruits of an international collaborative study (UEF, UCSF, CC, MIT) were recently published in the Journal of Orthopaedic Research.
Rupture of the anterior cruciate ligament is a typical sports injury. An injured knee joint can be treated with ligament reconstruction surgery which aims to restore the joint environment to a healthy state. However, this goal is not always achieved. Instead, the biomechanical loading may be elevated locally and the joint inflammation may cause swelling and aching of the knee. Both of these mechanisms also promote the degradation of articular cartilage located at the ends of bones, possibly leading to a crippling disease called osteoarthritis.
Currently, no cure exists for osteoarthritis. Thus, prevention of the disease progression is a prestigious aim, which can only be achieved if the disease mechanisms are understood and their effects can be predicted. Insightful predictions open doors for the design of new intervention strategies and evaluation of their efficacy. To tackle these challenges, researchers from the University of Eastern Finland (UEF), the University of California San Francisco (UCSF), Cleveland Clinic (CC), and Massachusetts Institute of Technology (MIT) now report on a novel workflow for predicting both biomechanical and, for the first time, the inflammation-related changes occurring in post-traumatic osteoarthritis.
The workflow consists of magnetic resonance imaging of ligament-reconstructed patients, clinical gait analysis, and subject-specific 3D computational models providing insights into time-dependent degradative changes in cartilage composition. By having access to the synovial fluid aspirated from the patients’ knees, the researchers could also include the clinical concentrations of inflammatory molecules into the computational models.
“When comparing the model predictions with quantitative magnetic resonance images at 1- and 3-year follow-up time points, we observed a striking similarity in locations of cartilage degeneration. Briefly, the biomechanical degradation due to tissue shearing occurred especially near chondral lesions, whereas the effect of inflammation was seen in wider areas,” says the paper’s first author, Postdoctoral Researcher Gustavo Orozco, Ph.D.
“Interestingly, our model also predicts that if the inflammation could be halted before becoming chronic, the recovery of the cartilage composition closer to healthy levels is possible. This scenario could be feasible with fast clearance of pro-inflammatory molecules as well as supplementing the synovial fluid with drugs prohibiting excessive cytokine activation,” adds Early-Stage Researcher Atte Eskelinen, M.Sc.
The long-term aim is to develop a new generation of computational mechanobiological models capable of predicting the effects of both biomechanical and biochemical osteoarthritis progression mechanisms for several years ahead. The new study is a milestone on that path, highlighting the feasibility of the inclusion of both gait analysis and synovial fluid samples into predictive numerical models.
“Our computational approach could also be enhanced in the future to include different rehabilitation protocols, like weight-loss, and anti-catabolic drugs, to guide the decision-making of health care professionals with numerical predictions," concludes Early-Stage Researcher Joonas Kosonen, M.Sc.
The study has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant No 713645. The study has also been funded by the Academy of Finland, strategic funding of the University of Eastern Finland, the Finnish Cultural Foundation, Instrumentarium Science Foundation, the Swedish Research Council, Maire Lisko Foundation, Sigrid Jusélius Foundation, Päivikki and Sakari Sohlberg Foundation, Maud Kuistila Memorial Foundation, Saastamoinen Foundation, the National Institutes of Health, and the American Orthopaedic Society of Sports Medicine.
For further information, please contact:
Postdoctoral Researcher Gustavo A. Orozco, tel: +358 50 3485018, gustavo.orozco@uef.fi
Early-Stage Researcher Atte S.A. Eskelinen, tel: +358 40 7483373, atte.eskelinen@uef.fi
Early-Stage Researcher Joonas P. Kosonen, tel: +358 50 3043474, joonas.kosonen@uef.fi
Senior Researcher Petri Tanska, tel: +358 50 364 2842, petri.tanska@uef.fi
Original article:
Gustavo A. Orozco, Atte S.A. Eskelinen, Joonas Kosonen, Matthew Tanaka, Mingrui Yang, Thomas Link, Benjamin Ma, Xiaojuan Li, Alan Grodzinsky, Rami K. Korhonen & Petri Tanska
Shear strain and inflammation-induced fixed charge density loss in the knee joint following ACL injury and reconstruction: a computational study. Journal of Orthopaedic Research (2021). DOI: 10.1002/jor.25177.
The article is available for download at: https://onlinelibrary.wiley.com/doi/full/10.1002/jor.25177


