New Diamyd Medical-licensed patent issued
Diamyd Medical (Nasdaq Stockholm First North, Ticker: DMYD B) announced today that the University of California, Los Angeles, UCLA, has been granted a key patent for a combo treatment for type 1 diabetes with GABA and preproinsulin or an immunogenic fragment thereof. Immunogenic fragments include c-peptide, proinsulin and other insulin molecules. This adds to Diamyd Medical’s patent estate of exclusively licensed intellectual property using GABA for treatment and interception of type 1 diabetes and inflammatory diseases including type 2 diabetes, metabolic syndrome and rheumatoid arthritis. The Company also exclusively licenses UCLA patents for GAD65 (a major autoantigen in type 1 diabetes) for which the last patent expires 2032.
“This new patent may very well bring substantial value to Diamyd Medical since various preproinsulin derived compounds are being developed by others as Antigen Based Therapies (ABTs) in parallel with Diamyd Medical’s development of the GAD-based ABT, Diamyd®, says Anders Essen-Möller, President and CEO of Diamyd Medical. “The diabetes vaccine Diamyd® is clearly today’s leading candidate ABT for type 1 diabetes but any synergistic reinforcement of its efficacy is important. Like in cancer therapy, incremental improvements by combining compounds that hit the disease from different angles, is likely the winning path forward in the forthcoming battle for this multibillion USD market.”
Diamyd Medical is collaborating with Professor Kenneth McCormick, the University of Alabama at Birmingham, in a GABA/Diamyd® combo trial in 75 recent onset type 1 diabetes patients, ages 4-18 years. Recruitment has been ongoing since March this year.
Gamma-Amino Butyric Acid (GABA) is a major neurotransmitter. GABA taken orally is widely considered safe with few side effects (Tian, 2011), and is available over the counter in the US. Diamyd® has been used in clinical studies with more than 1,000 patients and has shown a good safety profile. In a European Phase III study Diamyd® showed good clinical effect in several subgroups, and a limited overall 16% efficacy (p=0.10) in preserving endogenous insulin secretion. Diamyd® is easy to administer in any clinical setting.
Tian, Kaufman, et al showed that combining GABA with GAD-alum (Diamyd®), synergistically prolongs transplanted beta cell survival in an animal model for type 1 diabetes (PLoS One, 2011). More recently the same authors, (Tian, Kaufman et al, Diabetes, 2014), reported that combined treatment with GABA plus proinsulin synergistically restored normoglycemia and promoted beta cell replication in newly diabetic mice. In conclusion, GABA in combination with antigen based therapy (ABT) holds promise for type 1 diabetes intervention leading to restored or improved endogenous insulin production.
Evidence is accumulating that GABA is an important compound for treatment and prevention for diabetes and other inflammatory diseases. GABA lowers the production of pro-inflammatory cytokines and stimulates beta cell proliferation while it inhibits apoptosis. (Ligon, Diabetologia, 2007; Soltani, Proc Natl Acad Sci USA, 2011; Birnir, Amino Acids 2013; Wan, 2015).
Tian, Dang, Chen, Guan, Jin, Atkinson and Kaufman also showed that GABA regulates both the survival and replication of human beta cells. (Diabetes, 2013).
Abstracts from related scientific articles are included in this press release as appendix further below.
Ongoing studies with GABA and/or Diamyd® include:
- GABA/ DIAMYD® – COMBINING GABA WITH DIAMYD®
A placebo-controlled study, where Diamyd® is being tested in combination with GABA. The study comprises 75 patients between the ages of 4 and 18 recently diagnosed with type 1 diabetes, and will continue for a total of 12 months. The aim of the combination treatment is to preserve the body’s residual capacity to produce insulin. The study is led by Professor Kenneth McCormick at the University of Alabama at Birmingham, USA. The first patient was included in the study in March 2015.
- DIABGAD-1 – COMBINING DIAMYD® WITH IBUPROFEN AND VITAMIN D
A placebo-controlled study, where Diamyd® is being tested in combination with ibuprofen and vitamin D. The study comprises a total of 64 patients between the ages of 10 and 18 recently diagnosed with type 1 diabetes, and will continue for a total of 30 months. The aim of the combination treatment is to preserve the body’s residual capacity to produce insulin. The study runs at nine clinics in Sweden and is led by Professor Johnny Ludvigsson at Linköping University, Sweden. 15 month results from the study are due in the fourth quarter of 2015.
- DIAGNODE – DIAMYD® IN LYMPH GLANDS IN COMBINATION WITH VITAMIN D
An open label study, where Diamyd® is administered directly into lymph nodes in combination with treatment with vitamin D. The study comprises five patients between the ages of 18 and 30 newly diagnosed with type 1 diabetes, and will continue for a total of 30 months. The aim of the study is to evaluate the safety of the combination treatment and the effect on the immune system and the patients’ insulin producing capacity. The study is led by Professor Johnny Ludvigsson at Linköping University, Sweden. The first patient was included in the study in February 2015.
- EDCR IIa – COMBINING DIAMYD® WITH ETANERCEPT AND VITAMIN D
An open label study, where Diamyd® is combined with etanercept and vitamin D. The study comprises 20 patients between the ages of 8 and 18 who have been newly diagnosed with type 1 diabetes, and will continue for a total of 30 months. The aim of the study is to evaluate the safety of the combination treatment and the effect on the immune system and the patients’ insulin producing capacity. The study is led by Professor Johnny Ludvigsson at Linköping University, Sweden. The first patient was included in May 2015.
- DiAPREV-IT 1 – DIAMYD®
A placebo-controlled study, where Diamyd® is being tested in children at high risk of developing type 1 diabetes, meaning that they have been found to have an ongoing autoimmune process but do not yet have any clinical symptoms of diabetes. A total of 50 participants from the age of four have been enrolled in the study, which will last for five years. The aim of the study is to evaluate whether Diamyd® can delay or prevent the participants from presenting with type 1 diabetes. The study is led by Dr. Helena Elding Larsson at Lund University, Sweden. Five year results are expected at the end of 2016.
- DiAPREV-IT 2 – COMBINING DIAMYD® WITH VITAMIN D
A placebo-controlled study, where Diamyd® is being tested in combination with vitamin D in children at high risk of developing type 1 diabetes, meaning that they have been found to have an ongoing autoimmune process but do not yet have any clinical symptoms of diabetes. A total of 80 participants between the ages of 4 and 18 will be enrolled in the study, which will last for five years. The aim of the study is to evaluate whether Diamyd® can delay or prevent the participants from presenting with type 1 diabetes. The study is led by Dr. Helena Elding Larsson at Lund University, Sweden. The first patient was included in March 2015.
About Diamyd Medical
Diamyd Medical is dedicated to working toward a cure for type 1 diabetes and LADA. The Company’s projects include development of combination regimens with the GAD-based diabetes vaccine Diamyd® for arresting the destruction of insulin-producing beta cells. The Company exclusively licenses UCLA-rights to GAD65, the active ingredient in the vaccine, for which the last patent expires in 2032. Additionally, the Company exclusively licenses UCLA patents for using GABA for the treatment of diabetes and other inflammation-related conditions.
Diamyd Medical is one of the major shareholders in the stem cell company Cellaviva AB, which is establishing a Swedish commercial bank for private family saving of stem cells in umbilical cord blood and other sources of stem cells. Stem cells can be expected to be used in Personalized Regenerative Medicine (PRM), for example, to restore beta cell mass in diabetes patients where autoimmunity has been arrested.
Remium Nordic AB is the Company’s Certified Adviser.
APPENDIX
Diabetes Metab Syndr Obes. 2015 Feb 3;8:79-87. doi: 10.2147/DMSO.S50642. eCollection 2015.
GABAergic system in the endocrine pancreas: a new target for diabetes treatment.
Wan Y, Wang Q, Prud'homme GJ.
Excessive loss of functional pancreatic β-cell mass, mainly due to apoptosis, is a major factor in the development of hyperglycemia in both type 1 and type 2 diabetes (T1D and T2D). In T1D, β-cells are destroyed by immunological mechanisms. In T2D, while metabolic factors are known to contribute to β-cell failure and subsequent apoptosis, mounting evidence suggests that islet inflammation also plays an important role in the loss of β-cell mass. Therefore, it is of great importance for clinical intervention to develop new therapies. γ-Aminobutyric acid (GABA), a major neurotransmitter, is also produced by islet β-cells, where it functions as an important intraislet transmitter in regulating islet-cell secretion and function. Importantly, recent studies performed in rodents, including in vivo studies of xenotransplanted human islets, reveal that GABA exerts β-cell regenerative effects. Moreover, it protects β-cells against apoptosis induced by cytokines, drugs, and other stresses, and has anti-inflammatory and immunoregulatory activities. It ameliorates the manifestations of diabetes in preclinical models, suggesting potential applications for the treatment of diabetic patients. This review outlines the actions of GABA relevant to β-cell regeneration, including its signaling mechanisms and potential interactions with other mediators. These studies increase our understanding of the regenerative processes of pancreatic β-cells, and help pave the way for the development of regenerative medicine for diabetes.
Diabetes. 2014 Sep;63(9):3128-34. doi: 10.2337/db13-1385.
Combined therapy with GABA and proinsulin/alum acts synergistically to restore long-term normoglycemia by modulating T-cell autoimmunity and promoting β-cell replication in newly diabetic NOD mice.
Tian J, Dang H, Nguyen AV, Chen Z, Kaufman DL.
Antigen-based therapies (ABTs) fail to restore normoglycemia in newly diabetic NOD mice, perhaps because too few β-cells remain by the time that ABT-induced regulatory responses arise and spread. We hypothesized that combining a fast-acting anti-inflammatory agent with an ABT could limit pathogenic responses while ABT-induced regulatory responses arose and spread. γ-Aminobutyric acid (GABA) administration can inhibit inflammation, enhance regulatory T-cell (Treg) responses, and promote β-cell replication in mice. We examined the effect of combining a prototypic ABT, proinsulin/alum, with GABA treatment in newly diabetic NOD mice. Proinsulin/alum monotherapy failed to correct hyperglycemia, while GABA monotherapy restored normoglycemia for a short period. Combined treatment restored normoglycemia in the long term with apparent permanent remission in some mice. Proinsulin/alum monotherapy induced interleukin (IL)-4- and IL-10-secreting T-cell responses that spread to other β-cell autoantigens. GABA monotherapy induced moderate IL-10 (but not IL-4) responses to β-cell autoantigens. Combined treatment synergistically reduced spontaneous type 1 T-helper cell responses to autoantigens, ABT-induced IL-4 and humoral responses, and insulitis, but enhanced IL-10 and Treg responses and promoted β-cell replication in the islets. Thus, combining ABT with GABA can inhibit pathogenic T-cell responses, induce Treg responses, promote β-cell replication, and effectively restore normoglycemia in newly diabetic NOD mice. Since these treatments appear safe for humans, they hold promise for type 1 diabetes intervention.
Amino Acids. 2013 Jul;45(1):87-94. doi: 10.1007/s00726-011-1193-7. Epub 2011 Dec 13.
GABA is an effective immunomodulatory molecule.
Jin Z, Mendu SK, Birnir B.
In recent years, it has become clear that there is an extensive cross-talk between the nervous and the immune system. Somewhat surprisingly, the immune cells themselves do express components of the neuronal neurotransmitters systems. What role the neurotransmitters, their ion channels, receptors and transporters have in immune function and regulation is an emerging field of study. Several recent studies have shown that the immune system is capable of synthesizing and releasing the classical neurotransmitter GABA (γ-aminobutyric acid). GABA has a number of effects on the immune cells such as activation or suppression of cytokine secretion, modification of cell proliferation and GABA can even affect migration of the cells. The immune cells encounter GABA when released by the immune cells themselves or when the immune cells enter the brain. In addition, GABA can also be found in tissues like the lymph nodes, the islets of Langerhans and GABA is in high enough concentration in blood to activate, e.g., GABA-A channels. GABA appears to have a role in autoimmune diseases like multiple sclerosis, type 1 diabetes, and rheumatoid arthritis and may modulate the immune response to infections. In the near future, it will be important to work out what specific effects GABA has on the function of the different types of immune cells and determine the underlying mechanisms. In this review, we discuss some of the recent findings revealing the role of GABA as an immunomodulator.
Diabetes. 2013, 62:3760-5.
γ-Aminobutyric acid regulates both the survival and replication of human β-cells.
Tian J, Dang H, Chen Z, Guan A, Jin Y, Atkinson MA, Kaufman DL.
γ-Aminobutyric acid (GABA) has been shown to inhibit apoptosis of rodent β-cells in vitro. In this study, we show that activation of GABAA receptors (GABAA-Rs) or GABAB-Rs significantly inhibits oxidative stress-related β-cell apoptosis and preserves pancreatic β-cells in streptozotocin-rendered hyperglycemic mice. Moreover, treatment with GABA, or a GABAA-R- or GABAB-R-specific agonist, inhibited human β-cell apoptosis following islet transplantation into NOD/scid mice. Accordingly, activation of GABAA-Rs and/or GABAB-Rs may be a useful adjunct therapy for human islet transplantation. GABA-R agonists also promoted β-cell replication in hyperglycemic mice. While a number of agents can promote rodent β-cell replication, most fail to provide similar activities with human β-cells. In this study, we show that GABA administration promotes β-cell replication and functional recovery in human islets following implantation into NOD/scid mice. Human β-cell replication was induced by both GABAA-R and GABAB-R activation. Hence, GABA regulates both the survival and replication of human β-cells. These actions, together with the anti-inflammatory properties of GABA, suggest that modulation of peripheral GABA-Rs may represent a promising new therapeutic strategy for improving β-cell survival following human islet transplantation and increasing β-cells in patients with diabetes.
Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11692-7. doi: 10.1073/pnas.1102715108.
GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes.
Soltani N, Qiu H, Aleksic M, Glinka Y, Zhao F, Liu R, Li Y, Zhang N, Chakrabarti R, Ng T, Jin T, Zhang H, Lu WY, Feng ZP, Prud'homme GJ, Wang Q.
Type 1 diabetes (T1D) is an autoimmune disease characterized by insulitis and islet β-cell loss. Thus, an effective therapy may require β-cell restoration and immune suppression. Currently, there is no treatment that can achieve both goals efficiently. We report here that GABA exerts antidiabetic effects by acting on both the islet β-cells and immune system. Unlike in adult brain or islet α-cells in which GABA exerts hyperpolarizing effects, in islet β-cells, GABA produces membrane depolarization and Ca(2+) influx, leading to the activation of PI3-K/Akt-dependent growth and survival pathways. This provides a potential mechanism underlying our in vivo findings that GABA therapy preserves β-cell mass and prevents the development of T1D. Remarkably, in severely diabetic mice, GABA restores β-cell mass and reverses the disease. Furthermore, GABA suppresses insulitis and systemic inflammatory cytokine production. The β-cell regenerative and immunoinhibitory effects of GABA provide insights into the role of GABA in regulating islet cell function and glucose homeostasis, which may find clinical application.
PLoS One. 2011;6(9):e25337. doi: 10.1371/journal.pone.0025337. Epub 2011 Sep 22.
Combining antigen-based therapy with GABA treatment synergistically prolongs survival of transplanted ß-cells in diabetic NOD mice.
Tian J, Dang H, Kaufman DL.
Antigen-based therapies (ABTs) very effectively prevent the development of type 1 diabetes (T1D) when given to young nonobese diabetic (NOD) mice, however, they have little or no ability to reverse hyperglycemia in newly diabetic NOD mice. More importantly, ABTs have not yet demonstrated an ability to effectively preserve residual ß-cells in individuals newly diagnosed with type 1 diabetes (T1D). Accordingly, there is great interest in identifying new treatments that can be combined with ABTs to safely protect ß-cells in diabetic animals. The activation of γ-aminobutyric acid (GABA) receptors (GABA-Rs) on immune cells has been shown to prevent T1D, experimental autoimmune encephalomyelitis (EAE) and rheumatoid arthritis in mouse models. Based on GABA's ability to inhibit different autoimmune diseases and its safety profile, we tested whether the combination of ABT with GABA treatment could prolong the survival of transplanted ß-cells in newly diabetic NOD mice. Newly diabetic NOD mice were untreated, or given GAD/alum (20 or 100 µg) and placed on plain drinking water, or water containing GABA (2 or 6 mg/ml). Twenty-eight days later, they received syngenic pancreas grafts and were monitored for the recurrence of hyperglycemia. Hyperglycemia reoccurred in the recipients given plain water, GAD monotherapy, GABA monotherapy, GAD (20 µg)+GABA (2 mg/ml), GAD (20 µg)+GABA (6 mg/ml) and GAD (100 µg)+GABA (6 mg/ml) about 1, 2-3, 3, 2-3, 3-8 and 10-11 weeks post-transplantation, respectively. Thus, combined GABA and ABT treatment had a synergistic effect in a dose-dependent fashion. These findings suggest that co-treatment with GABA (or other GABA-R agonists) may provide a new strategy to safely enhance the efficacy of other therapeutics designed to prevent or reverse T1D, as well as other T cell-mediated autoimmune diseases.
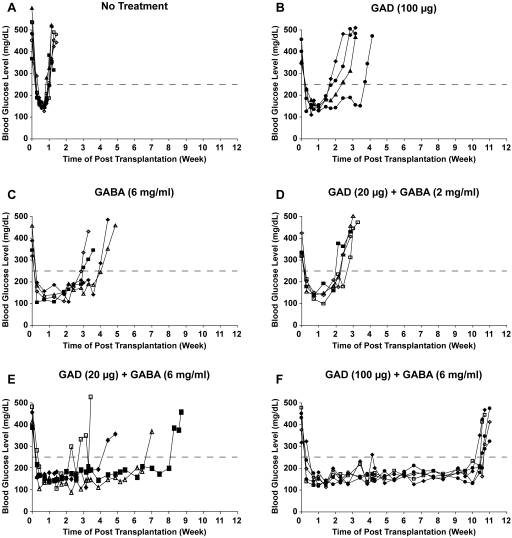
Fig 1. Synergistic effects of combined GAD/alum+GABA treatment to prolong transplanted syngenic ß-cell survival in diabetic NOD mice (Tian 2011 e25338)
Diabetologia. 2007 Apr;50(4):764-73. Epub 2007 Feb 22.
Regulation of pancreatic islet cell survival and replication by gamma-aminobutyric acid.
Ligon B, Yang J, Morin SB, Ruberti MF, Steer ML.
AIMS/HYPOTHESIS:
Pancreatic islets have evolved remarkable, though poorly understood mechanisms to modify beta cell mass when nutrient intake fluctuates or cells are damaged. We hypothesised that appropriate and timely adjustments in cell number occur because beta cells release proliferative signals to surrounding cells when stimulated by nutrients and 'bleed' these growth factors upon injury.
MATERIALS AND METHODS:
In rat pancreatic islets, we measured DNA content, insulin content, insulin secretion after treatment, immunoblots of apoptotic proteins and the uptake of nucleoside analogues to assess the ability of gamma-aminobutyric acid (GABA), which is highly concentrated in beta cells, to act as a growth and survival factor. This focus is supported by work from others demonstrating that GABA increases cell proliferation in the developing nervous system, acts as a survival factor for differentiated neurons and, interestingly, protects plants under stress.
RESULTS:
Our results show that DNA, insulin content and insulin secretion are higher in freshly isolated islets treated with GABA or GABA B receptor agonists. Exposure to GABA upregulated the anti-apoptotic protein B-cell chronic lymphocytic leukaemia XL and limited activation of caspase 3 in islets. The cellular proliferation rate in GABA-treated islets was twice that of untreated controls.
CONCLUSIONS/INTERPRETATION:
We conclude that GABA serves diverse purposes in the islet, meeting a number of functional criteria to act as an endogenous co-regulator of beta cell mass.
For further information, please contact:
Anders Essen-Möller, President and CEO
Phone: +46 70 55 10 679. E-mail: anders.essen-moller@diamyd.com
Diamyd Medical AB (publ)
Kungsgatan 29, SE-111 56 Stockholm, Sweden. Phone: +46 8 661 00 26, Fax: +46 8 661 63 68
E-mail: info@diamyd.com. Reg. no.: 556242-3797. Website: www.diamyd.com.
Tags:

