Quarterly Report I 15/16
September 2015 – November 2015
Diamyd Medical AB (publ), Fiscal year 2015/2016
Reporting period, September 1, 2015 – November 30, 2015
- Net result amounted to MSEK -4.3 (-5.9)
- Net result per share amounted to SEK -0.2 (-0.3)
- Cash flow from operating activities amounted to MSEK -4.8 (-5.8)
- Liquid assets and short term investments amounted at the end of the period to MSEK 24.9 (29.8)
Significant events after the reporting period
- Preliminary 15-month results from DIABGAD, a 30-month pilot study with the diabetes vaccine Diamyd® in combination with vitamin D and ibuprofen, were reported
- Diamyd Medical plans first interim report from open label pilot study EDCR IIa with the diabetes vaccine Diamyd® in the first quarter of 2016
CEO comments
Dear Shareholders,
We take great pleasure in reporting that Diamyd Medical continues to strengthen its position as a world leader in the development of Antigen Based Therapy for type 1 diabetes. A positive trend for the Diamyd® therapy could be reported from the end of the first 15-month period of the ongoing 30-month DIABGAD study. Limited six-month findings from the EDCR study will soon be available, as well as limited six-month findings from the DIAGNODE study. Particularly pleasing is the growing interest shown by the pharmaceutical industry in our approach to combining GABA with antigen-based therapy for autoimmune diseases, not limited to type 1 diabetes, and for certain inflammatory disorders.
A complete cure for type 1 diabetes could be expected to include: A) manipulation of the immune response to facilitate susceptibility to tolerance induction; B) introduction of immunological tolerance to the specific antigens exposed to the autoimmune attack, and C) increasing the functional beta cell mass to enable sufficient endogenous insulin secretion.
A possible scenario is that, while several companies may develop A and C components, Diamyd Medical will be first to market with a B component, and then continue to further improve on its antigen-specific therapy.
We are very excited about being the leading company in terms of component B development, i.e. the induction of tolerance to specific beta cell antigens. Several clinical trials have been carried out with GAD65 as the beta cell antigen. In a Phase III trial, the diabetes vaccine Diamyd® (GAD in alum) demonstrated 16 percent (p=0.1) higher efficacy than a placebo in terms of the ability to produce insulin after 15 months. Diamyd® has demonstrated a good safety profile in trials with more than 1,000 patients.
We believe that our B medication component (the diabetes vaccine Diamyd®) can be granted market approval as soon as the tolerance-inducing effect of the diabetes vaccine can be enhanced, preferably through a combination with an existing and market-approved A component. Think cancer – a combination of medications usually provides the best treatment outcomes.
There is a steadily growing stream of possible A and C medications that through non-specific mechanisms could enhance the tolerance-inducing ability of antigen-specific therapy for type 1 diabetes. Most of these could in principle be used in combination with the diabetes vaccine Diamyd®. Examples of such molecules include GLP-1 analogues, DPP4 inhibitors, metformin, granulocyte colony stimulating factor (GCSF), antithymocyte globulin (ATG), abatacept, alefacept, demethylation agents, the IL-10 cytokine, ustekinumab (Stelara) and antibodies against T and B blood cells. We have chosen to study Diamyd® in combination with some A components, such as ibuprofen, etanercept, vitamin D and GABA (the last-mentioned for which, as previously reported by Diamyd Medical, interesting pre-clinical proof of concept has been shown, see the figure below).
Although our antigen-specific drug candidate, Diamyd®, is the leader in its class, and could be approved as a separate medication in combination with a suitable A component, a potential Diamyd 2.0 is currently under development, in where additional antigens such as gliadin and/or proinsulin may be introduced to broaden the patient population, in which Diamyd®, can possibly stop the autoimmune attack on beta cells.
We are also studying the effect of various administration methods for the diabetes vaccine Diamyd®, such as subcutaneous injection in the arm, subcutaneous injection in the abdomen (closer to the pancreatic draining lymph nodes) and directly into lymph nodes. Pre-clinical testing is ongoing with the ex-vivo co-culturing of antigens and tolerant antigen-presenting cells (APCs), with the goal of gradually injecting them back into patients (cell therapy).
Once the autoimmune attack has been overcome, the beta cell mass must be increased so that individuals with type 1 diabetes can produce enough insulin on their own. There are several strategies for such C components: transplantation of insulin-producing cells combined with immunosuppression to prevent rejection; transplantation of insulin-producing cells that are encapsulated to avoid being recognized as foreign tissue; autologous transplantation of stem cell-derived beta cells, and administration of beta cell regeneration components, such as e.g. GABA molecules.
Current development pipeline - Pre-clinical proof-of-concept
Combination therapy using GABA and antigen (GAD65) SELECTED RESULTS
GABA + GAD/alum combination therapy shows significant synergistic effects on normoglycemia in NOD mice
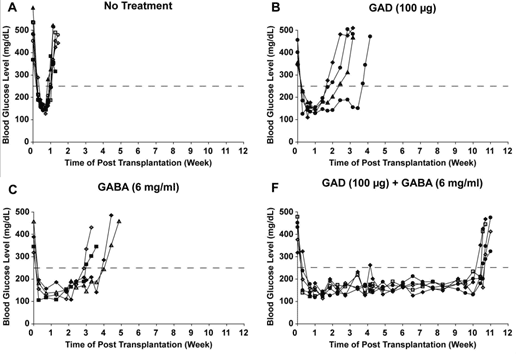
REFERENCES: Tian, Jide, Hoa Dang, and Daniel L. Kaufman. "Combining Antigen-Based Therapy with GABA Treatment Synergistically Prolongs Survival of Transplanted ß-Cells in Diabetic NOD Mice." PLoS ONE, 2011.
Current development pipeline - Pre-clinical proof-of-concept
Combination therapy using GABA and antigen (Proinsulin) SELECTED RESULTS
GABA + Proinsulin/alum combination therapy shows significant synergistic effects on normoglycemia in NOD mice
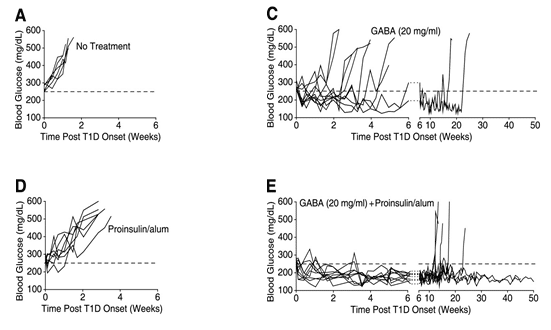
REFERENCES: Tian, J., H. Dang, A. V. Nguyen, Z. Chen, and D. L. Kaufman. "Combined Therapy With GABA and Proinsulin/Alum Acts Synergistically to Restore Long-term Normoglycemia by Modulating T-Cell Autoimmunity and Promoting -Cell Replication in Newly Diabetic NOD Mice." Diabetes, 2014, 3128-134.
A new calendar year has begun. One thing is certain – it will be an eventful year. We are looking forward to working hard – with the goal of generating value for our shareholders, and for those with type 1 diabetes.
Stockholm, January 20, 2016
Anders Essen-Möller
President and CEO Diamyd Medical AB (publ)
Significant events after the reporting period
Preliminary 15-month results from DIABGAD – a 30-month pilot study with the diabetes vaccine Diamyd® in combination with vitamin D and ibuprofen
Diamyd Medical announced from a clinical investigator-initiated pilot study, DIABGAD-1, that the diabetes vaccine Diamyd® in combination with vitamin D and ibuprofen after 15 months has a good safety profile with no reported serious side effects related to the treatment. The data shows that after the initial phase (referred to partial remission or the honeymoon phase), the group receiving placebo (non-active substance) lost their ability to produce insulin at a rate that was 2-3 times faster during the last 9 months of the 15-month period, compared with the groups receiving active treatment with Diamyd®. However, viewed over the entire 15-month period no difference between the groups is observed, but if the more rapid decrease continues in the placebo group until the end of the study at 30 months, a trend deviation in insulin production may be observable throughout the full measurement period, that is, including the remission period.
Diamyd Medical plans first interim report from open label pilot study EDCR IIa with the diabetes vaccine Diamyd® in the first quarter of 2016
Diamyd Medical announced that a first six month interim report comprising five patients treated with etanercept and the diabetes vaccine Diamyd® is intended to be presented during the first quarter of 2016. The study is open labeled, meaning not placebo-controlled, and is conducted at nine pediatric diabetes clinics in Sweden. Professor Johnny Ludvigsson, Linköping University is principal investigator and sponsor of the study.
Antigen Based Therapy (ABT) and combination trials
Type 1 diabetes is a devastating disease which requires daily treatment with insulin to sustain life. The importance of finding a cure should not be underestimated. The diabetes vaccine Diamyd® has been used in clinical studies with more than 1,000 patients and has shown a good safety profile. In a European Phase III trial Diamyd® showed good clinical effect in several subgroups, and a limited overall 16% efficacy (p=0.10) in preserving endogenous insulin secretion. Subsequent development is focused on combination trials to enhance efficacy. Diamyd® is easy to administer in any clinical setting. The potential annual market is estimated to several billion dollars per year.
Six researcher initiated clinical trials are ongoing combining Diamyd® with various other immunomodulatory compounds; etanercept, ibuprofen, vitamin D and GABA.
- DIABGAD- 1 – COMBINING DIAMYD® WITH IBUPROFEN AND VITAMIN D
INTERVENTION TRIAL
A placebo-controlled trial, where Diamyd® is being tested in combination with ibuprofen and vitamin D. The trial comprises a total of 64 patients between the ages of 10 and 18, recently diagnosed with type 1 diabetes, and will continue for a total of 30 months. The aim of the combination treatment is to preserve the body’s own capacity to produce insulin. The trial runs at nine clinics in Sweden and is led by Professor Johnny Ludvigsson at Linköping University, Sweden. 30 month results from the trial are due during the first half year of 2017.
- DIAGNODE -1 –DIAMYD® IN LYMPH GLANDS IN COMBINATION WITH VITAMIN D
INTERVENTION TRIAL
An open label trial, where Diamyd® is administered directly into lymph nodes in combination with treatment with vitamin D. The trial comprises five patients between the ages of 18 and 30 newly diagnosed with type 1 diabetes, and will continue for a total of 30 months. The aim of the trial is to evaluate the safety of the combination treatment and the effect on the immune system and the patients’ insulin producing capacity. The trial is led by Professor Johnny Ludvigsson at Linköping University, Sweden. The first patient was included in the trial in February 2015. A first interim report is planned in the first quarter of 2016.
- GABA/ DIAMYD® – COMBINING DIAMYD® WITH GABA
INTERVENTION TRIAL
A placebo-controlled trial, where Diamyd® is being tested in combination with GABA. The trial comprises 75 patients between the ages of 4 and 18 recently diagnosed with type 1 diabetes, and will continue for a total of 12 months. The aim of the combination treatment is to preserve the body’s residual capacity to produce insulin. The trial is led by Professor Kenneth McCormick at the University of Alabama at Birmingham, USA. The first patient was included in the trial in March 2015.
- EDCR IIa – COMBINING DIAMYD® WITH ETANERCEPT AND VITAMIN D
INTERVENTION TRIAL
An open label trial, where Diamyd® is combined with etanercept and vitamin D. The trial comprises 20 patients between the ages of 8 and 18 who have been newly diagnosed with type 1 diabetes, and will continue for a total of 30 months. The aim of the trial is to evaluate the safety of the combination treatment and the effect on the immune system and the patients’ insulin producing capacity. The trial is led by Professor Johnny Ludvigsson at Linköping University, Sweden. The first patient was included in May 2015. A first interim report is planned in the first quarter of 2016.
- DiAPREV-IT 1– DIAMYD®
PREVENTION TRIAL
A placebo-controlled trial, where Diamyd® is being tested in children at high risk of developing type 1 diabetes, meaning that they have been found to have an ongoing autoimmune process but do not yet have any clinical symptoms of diabetes. A total of 50 participants from the age of four have been enrolled in the trial, which will last for five years. The aim of the trial is to evaluate whether Diamyd® can delay or prevent the participants from presenting with type 1 diabetes. The trial is led by Dr. Helena Elding Larsson at Lund University, Sweden. Five year results are expected at the end of 2016.
- DiAPREV-IT 2 – COMBINING DIAMYD® WITH VITAMIN D
PREVENTION TRIAL
A placebo-controlled trial, where Diamyd® is being tested in combination with vitamin D in children at high risk of developing type 1 diabetes, meaning that they have been found to have an ongoing autoimmune process but do not yet have any clinical symptoms of diabetes. A total of 80 participants between the ages of 4 and 18 will be enrolled in the trial, which will last for five years. The aim of the trial is to evaluate whether Diamyd® can delay or prevent the participants from presenting with type 1 diabetes. The trial is led by Dr. Helena Elding Larsson at Lund University, Sweden. The first patient was included in March 2015.
*** The above is an excerpt from the report. To read the complete report, please visit www.diamyd.com, or see attached PDF ***
For more information please contact:
Anders Essen-Möller, President and CEO, phone: +46 70 55 10 679
Diamyd Medical AB (publ), Kungsgatan 29, SE-111 56 Stockholm, Sweden
Phone: +46 8 661 00 26 Fax: +46 8 661 63 68 E-mail: info@diamyd.com Reg. no: 556242-3797
Note: This document has been prepared in both Swedish and English. The Swedish version shall govern in case of differences between the two documents. The document contains certain statements about the Company’s operating environment and future performance. These statements should only be regarded as reflective of prevailing interpretations. No guarantees can be made that these statements are free from errors.
Tags:

