PolarCool AB (publ) has submitted a joint application for Eurostars grant, together with its partner ABCDx and Lund University, which could generate 1 MEUR for the consortium
PolarCool, ABCDx SA, and Lund University have submitted a joint application for EU grants through the Eurostars program. The application could generate up to 1 million Euro in EU grants for the consortium. Eurostars is an EU program which supports international innovative projects led by research and development performing small- and medium-sized enterprises (SMEs). The program has EUR 57 million annually available for co-financing the development of new products and technologies.
The consortium will develop and commercialize PolarTBICheck™, a portable cost-effective solution for early diagnosis and treatment of sports-related concussions. Given the positive existing evidence base for TBICheck™ and PolarCap® System, we are confident that the integration of both products will be an attractive and unique product for athletes and their medical directors.
More than 25 million people annually suffer from a concussion, of which 10 million visit the hospital for a CT examination while the other 15 million are not examined due to the high costs that associated with CT examinations. Particularly in sports medicine, concussions have become a major health problem that causes short- and long-term problems such as headaches, nausea, sound and light sensitivity, depression, and neurodegenerative diseases. Repeated sub-concussive head collisions can also lead to chronic brain damage such as Chronic Traumatic Encephalopathy (CTE).
Traumatic brain injury contributes to about one-third of all injury-related deaths and represents a significant financial burden ($ 76.5 billion in the United States alone). Cycling, American football, soccer, martial arts, rugby, and ice hockey are the sports where practitioners are most at risk of brain injury.
TBICheck™ is a new rapid blood finger point-of-care test for early diagnostic and decision process. The tool measures a panel of biomarkers in the blood. Within 15 minutes of taking a blood sample, TBICheck™ will provide a diagnostic result, with high precision, determining the risk of incurred brain injury. Within the healthcare sector, TBICheck™ can mean a large cost savings as many CT examinations can be avoided. Currently, the biomarker S100B is an established method for decision process concerning examinations of light and moderate head injuries (Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: an evidence and consensus-based update. Undén J, Ingebrigtsen T, Romner B). According to studies referred to by ABCDx, the combination H-FABP and GFAP has proven to be more effective than the established marker S100B.
TBICheck™ can potentially be used within sports, assisting medical staff in determining whether or not a concussion has occurred. It can also be used as a supplement to clinical diagnostics, as well as to evaluate whether a player needs to start rehabilitation or not. Within the military, where brain injury can incur far from the healthcare facilities, the product can be applied to evaluate whether an injured individual need to seek medical care or not.
In collaboration with the Swedish Hockey League (SHL) and HockeyAllsvenskan, PolarCool conducted a study during the past three seasons, wherein PolarCap® System has been evaluated for the treatment of concussion. The results show that cooling with PolarCap® System has been advantageous in the treatment of concussion. A total of 80 cases were reported in the study. The median time for return to play for the intervention group, treated with PolarCap® System, was 7 days and for those not treated 12 days. The results are statistically significant (p < 0.001). In addition, more players in the control group (12 of 51; 24%) were absent from games longer than 3 weeks, compared to the intervention group (2 of 29; 7%). These results are also statistically significant (p = 0.028).
At the latest board meeting, held on September 26, 2019, PolarCool decided to begin the development of a mobile application (APP) to be used primarily as support for the athlete during the rehabilitation amidst a mild traumatic brain injury (mTBI) and concussion-related diseases. The APP development is intended to be conducted in collaboration with an IT partner. Within the framework of the consortium, both ABCDx and PolarCool will develop mobile applications that will become integrated tools to act as important support for doctors and athletes in the diagnosis and treatment of mild traumatic brain injury (mTBI).
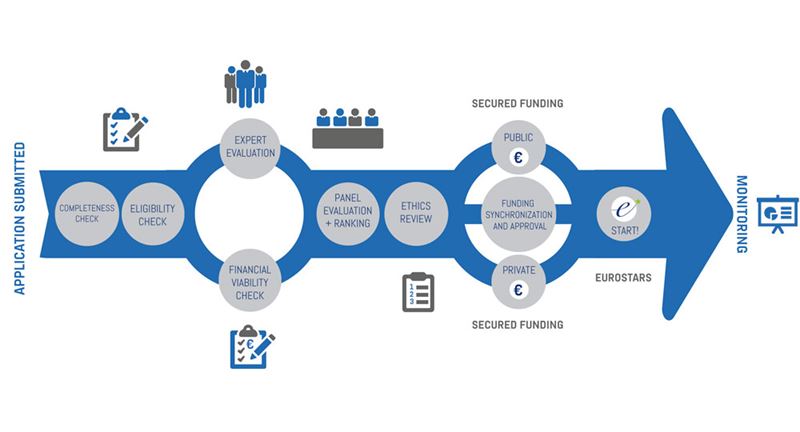
The consortium's application has now passed an important milestone in step 2, the so-called "eligibility stage". The selection process is shown below. All applications that have passed the eligibility stage are currently being considered by an expert panel and within 4 months of submission, answers are expected to be announced to those who are awarded funds and selected as winners in Eurostars. Thus, towards the end of January 2020 PolarCool expects to be notified of the outcome.
The entire evaluation process is described in detail in this link: https://www.eurostars-eureka.eu/eurostars-process-evaluation
For more information
Matz Johansson – CEO PolarCool AB (publ)
+46 - 731 45 14 93
E-mail: matz.johansson@polarcool.se
About PolarCool AB (publ)
PolarCool AB (publ) is a medical device company that develops, markets, and sells products for sports medicine. The company focuses on treatment of concussive and sub-concussive brain injury with the portable cooling device PolarCap® System. PolarCool AB (publ) is based in Lund, Sweden, and its shares are listed on Spotlight Stock Market.
Tags:


