Ultomiris demonstrated sustained improvements in functional activities and quality of life in adults with generalised myasthenia gravis through 60 weeks
Analysis of Phase III CHAMPION-MG trial open-label extension adds to growing body of safety and efficacy data for Ultomiris in generalised myasthenia gravis. Patients who transitioned from placebo to Ultomiris showed rapid and sustained response, reinforcing clinical benefit of C5 inhibition.
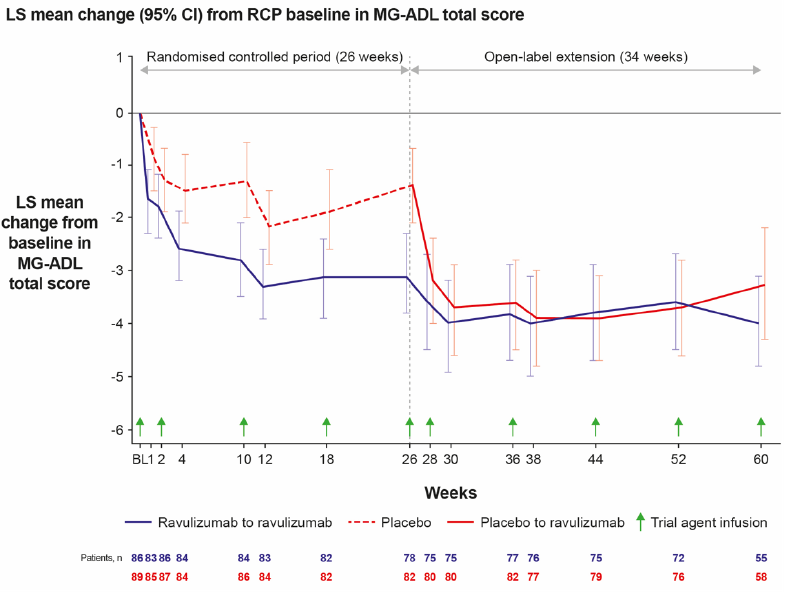
New, prolonged follow-up results from the Phase III CHAMPION-MG trial open-label extension (OLE) showed that Ultomiris (ravulizumab-cwvz) demonstrated long-term efficacy in adults with anti-acetylcholine receptor (AChR) antibody-positive generalised myasthenia gravis (gMG), with improvements in activities of daily living, muscle strength and quality of life, sustained through 60 weeks.1 Ultomiris was also well tolerated throughout this analysis.
Results from the trial were presented on 5 April at the 2022 American Academy of Neurology (AAN) Annual Meeting.
gMG is a rare, debilitating, chronic, autoimmune neuromuscular disease that leads to a loss of muscle function and severe weakness.2 Symptoms of gMG may include disabling fatigue, slurred speech, difficulty swallowing and eating, double or blurred vision, immobility requiring assistance, shortness of breath, and episodes of respiratory failure.3-5
Professor James F. Howard, Jr, MD, Department of Neurology at The University of North Carolina School of Medicine and lead primary investigator in the CHAMPION-MG trial said: “gMG is a complex, devastating disease, disrupting many aspects of daily living, and helping patients improve muscle strength and function should be essential to any treatment plan. These results reinforce that C5 inhibition with predictable dosing is an important treatment option which provides sustained improvement of functional activities.”
Gianluca Pirozzi, MD, PhD, Senior Vice President, Head of Development and Safety, Alexion, said: “Alexion has pioneered the research of complement inhibition as a treatment approach for rare diseases, and we are continuing to innovate to benefit as many patients as possible. These data are encouraging because they suggest Ultomiris has the potential to help a broader range of gMG patients, including those with milder symptoms, regain control of their lives and experience sustained clinical benefit through 60 weeks. We are deeply grateful for continued input and collaboration from the gMG community.”
Upon completion of the randomised control period (RCP) of the CHAMPION-MG trial, 99.4% of participants (n=161) entered the OLE, during which all patients received Ultomiris. At the time of data cut off, 113 patients had reached 60 weeks. Efficacy analysis included all patients who received ≥1 dose of Ultomiris in the OLE.1
Ultomiris demonstrated statistically significant improvements from baseline (defined as initiation of Ultomiris therapy) in measures of functional activity, muscle strength and quality of life at 60 weeks of the OLE, including Myasthenia Gravis-Activities of Daily Living (MG-ADL) total score (-4.0 [95% CI -4.8, -3.1], p<0.0001). Additionally, patients transitioning from placebo (n=83) showed rapid response at a similar magnitude and time course as those who received Ultomiris during the RCP.1
Summary of efficacy results
Changes in scores from RCP baseline among patients who received Ultomiris for 60 weeksi,ii
|
|
Analysis at week 60 of Ultomiris treatment (n=78) |
|
MG-ADL Total Score |
-4.0 [95% CI -4.8, -3.1], p<0.0001 |
|
Quantitative Myasthenia Gravis Total Score |
-4.1 [95% CI -5.4, -2.9], p<0.0001 |
|
Revised 15-Item Myasthenia Gravis Quality of Life Score |
-5.0 [95% CI -6.9, -3.1], p<0.0001 |
|
Neurological Quality of Life Fatigue Subscale Score |
-10.2 [95% CI -15.1, -5.3], p<0.0001 |
- Results analysed using mixed-effect model for repeated measures, reported as least squares mean change from RCP baseline
- Eleven patients discontinued OLE prior to the data cut-off
The safety and tolerability were consistent with the known safety profile of Ultomiris observed in the RCP of CHAMPION-MG and other approved indications. The most common adverse events (AEs) (occurring in greater than or equal to 10% of 169 patients treated with Ultomiris in the RCP and/or OLE) were headache (16.6%) and diarrhoea (13.6%).1
Additional results from the CHAMPION-MG trial and pharmacokinetics and pharmacodynamics of Ultomiris in gMG were also presented at the 2022 AAN Annual Meeting.
Regulatory submissions for Ultomiris for the treatment of gMG are currently under review with multiple health authorities, including in the United States (US), European Union (EU) and Japan.
Notes
gMG
gMG is a rare autoimmune disorder characterised by loss of muscle function and severe muscle weakness.2
85% of people with gMG are AChR Ab+, meaning they produce specific antibodies (anti-AChR) that bind to signal receptors at the neuromuscular junction (NMJ), the connection point between nerve cells and the muscles they control. This binding activates the complement system, which is essential to the body’s defense against infection, causing the immune system to attack the NMJ.2 This leads to inflammation and a breakdown in communication between the brain and the muscles.2
gMG can occur at any age, but most commonly begins for women before the age of 40 and for men after the age of 60.6-8 Initial symptoms may include slurred speech, double vision, droopy eyelids, and lack of balance; these can often lead to more severe symptoms as the disease progresses such as, impaired swallowing, choking, extreme fatigue, and respiratory failure.4,9
CHAMPION-MG
The global Phase III randomised, double-blind, placebo-controlled, multicentre 26-week trial evaluated the safety and efficacy of Ultomiris in adults with gMG who were not previously treated with a complement inhibitor medicine. The trial enrolled 175 patients across North America, Europe, Asia-Pacific and Japan. Participants were required to have a confirmed myasthenia gravis diagnosis at least six months prior to the screening visit with a positive serologic test for anti-AChR antibodies, MG-ADL total score of at least 6 at trial entry and Myasthenia Gravis Foundation of America Clinical Classification Class II to IV at screening. There was no requirement for prior treatment failure, and patients could stay on stable standard of care medicines, with a few exceptions, for the duration of the randomised control period.10
Patients were randomised 1:1 to receive Ultomiris or placebo for a total of 26 weeks. Patients received a single weight-based loading dose on Day 1, followed by regular weight-based maintenance dosing beginning on Day 15, every eight weeks. The primary endpoint of change from baseline in the MG-ADL total score at Week 26 was assessed along with multiple secondary endpoints evaluating improvement in disease-related and quality-of-life measures.
Patients who completed the randomised control period were eligible to continue into an open-label extension period evaluating the safety and efficacy of Ultomiris, which is ongoing.
Ultomiris
Ultomiris (ravulizumab-cwvz), the first and only long-acting C5 complement inhibitor, offers immediate, complete and sustained complement inhibition. The medication works by inhibiting the C5 protein in the terminal complement cascade, a part of the body’s immune system. When activated in an uncontrolled manner, the complement cascade over-responds, leading the body to attack its own healthy cells. Ultomiris is administered intravenously every eight weeks in adult patients, following a loading dose.
Ultomiris is approved in the US, EU and Japan for the treatment of certain adults and children with paroxysmal nocturnal haemoglobinuria (PNH) based on the ALXN1210-PNH-302 and ALXN1210-PNH-304 Phase III trials.
Additionally, Ultomiris is approved in the US, EU and Japan for certain adults and children with atypical haemolytic uraemic syndrome (aHUS) based on the ALXN1210-aHUS-311 and ALXN1210-aHUS-312 Phase III trials.
As part of a broad development programme, Ultomiris is being assessed for the treatment of additional haematology and neurology indications.
Alexion
Alexion, AstraZeneca Rare Disease, is the group within AstraZeneca focused on rare diseases, created following the 2021 acquisition of Alexion Pharmaceuticals, Inc. As a leader in rare diseases for nearly 30 years, Alexion is focused on serving patients and families affected by rare diseases and devastating conditions through the discovery, development and commercialisation of life-changing medicines. Alexion focuses its research efforts on novel molecules and targets in the complement cascade and its development efforts on haematology, nephrology, neurology, metabolic disorders, cardiology and ophthalmology. Headquartered in Boston, Massachusetts, Alexion has offices around the globe and serves patients in more than 50 countries.
AstraZeneca
AstraZeneca (LSE/STO/Nasdaq: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development, and commercialisation of prescription medicines in Oncology, Rare Diseases, and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. Please visit astrazeneca.com and follow the Company on Twitter @AstraZeneca.
Contacts
For details on how to contact the Investor Relations Team, please click here. For Media contacts, click here.
References
- Howard, JF., Vu, T., Mantegazza, R., et al. Long-term efficacy and safety of ravulizumab, a long-acting terminal complement inhibitor, in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: Results from the phase 3 CHAMPION MG open-label extension. Oral presentation at: American Academy of Neurology (AAN) Annual Meeting; April 5, 2022; Session S25.005.
- Howard, J. F., (2017). Myasthenia gravis: the role of complement at the neuromuscular junction. Annals of The New York Academy of Sciences, 1412(1), 113-128.
- Howard JF, Barohn RJ, Cutter GR, et al. A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve. 2013;48(1):76-84.
- Myasthenia Gravis Fact Sheet. (2020, April 27). National Institutes of Neurological Disorders and Stroke. Available here. Accessed March 2022.
- Sathasivam S. Diagnosis and management of myasthenia gravis. Wiley Online Library website. https://onlinelibrary.wiley.com/doi/pdf/10.1002/pnp.315. Accessed September 25, 2019.
- Myasthenia Gravis. National Organization for Rare Disorders (NORD). Available here. Accessed March 2022.
- Howard, J. F. (2015). Clinical Overview of MG. Available here. Accessed March 2022.
- Sanders, D. B., Raja, S. M., Guptill J. T., Hobson-Webb, L. D., Juel, V. C., & Massey, J. M. (2020). The Duke myasthenia gravis clinic registry: I. Description and demographics. Muscle & Nerve, 63(2), 209-216.
- Ding J., Zhao, S., Ren, K., Dang, D., Li, H., Wu, F., Zhang, M., Li, Z., & Guo, J. (2020). Prediction of generalization of ocular myasthenia gravis under immunosuppressive therapy in Northwest China. BMC Neurology, 20(238).
- ClinicalTrials.gov. Safety and Efficacy Study of Ravulizumab in Adults With Generalized Myasthenia Gravis. NCT Identifier: NCT03920293. Available online. Accessed March 2022.


