BrainCool AB (publ): New Scientific Publication of the RhinoChill[®] System in Critical Care with significant results
A new publication in Critical Care, https://ccforum.biomedcentral.com/articles/10.1186/s13054-021-03583-9), shows statistically significant results on patients with initial non-shockable rhythms, with the product RhinoChill®. The scientific publication is a pooling of data of the two clinical studies, Prince and Princess (studies conducted with the BrainCool’s product, RhinoChill® System. The publication authored by the research investigators of the PRINCESS trial (Dr. Fabio Silvio Taccone et al), Dr. Taccone, is one of ERC key opinion leader in the field of post resuscitation care.
The pooled analysis of 858 patients from two randomized trials (PRINCE and PRINCESS) assessing the effect of prehospital trans nasal evaporative intra-arrest cooling in OHCAs. In the analysis, a total of 843 patients were included of which 325 patients had initial shockable rhythms (158 intervention vs. 167 control) and 518 had initial non-shockable rhythms, asystole or pulseless electric activity (250 intervention vs. 267 control). The non-shockable rhythm patients, is a patient group with high mortality before even reaching to the hospital.Among patients with initial shockable rhythm, the results showed a statistically significant result (p-value = 0,027) in the patients with shockable rhythm, with a survival with CPC 1-2 at 90 days of 36,4 % in the active group (cooling with RhinoChill® from the ambulance) compared to the 25,6 % in the control group (cooling only at the ICU). In November 2020, American Heart Association’s guidelines have been updated and strongly advised to implement early cooling and advanced TTM ("target temperature management") systems following cardiac arrest.
CEO Martin Waleij comments;
”The key to an effective treatment of hypothermia is reaching target temperature as quickly as possible. As seen in the time analysis publication [1], targeted temperature management leads to improved results with initiation of treatment as early as possible.
This is yet another piece of evidence for an urgent need for early-stage medical cooling solutions allowing cooling initiation as early as possible in shockable rhythm OHCA patients. The current high rates of mortality and neurological disability following cardiac arrest constitute a great opportunity to make a difference, as well as a great business opportunity for BrainCool. BrainCool’s envision is combining early on-site treatment by RhinoChill® with an automatic and smooth transition to the ICU cooling treatment by BrainCool™/ IQool™ for the continuum optimal care”.
This gives an indication of the interest from this new method, which is the only safe and feasible method to implement cooling treatment within minutes from the onset of a cardiac arrest. New guidelines in Europe (European Resuscitation Council), and the US (American Heart) have confirmed that cooling treatment shall be started as soon as possible.
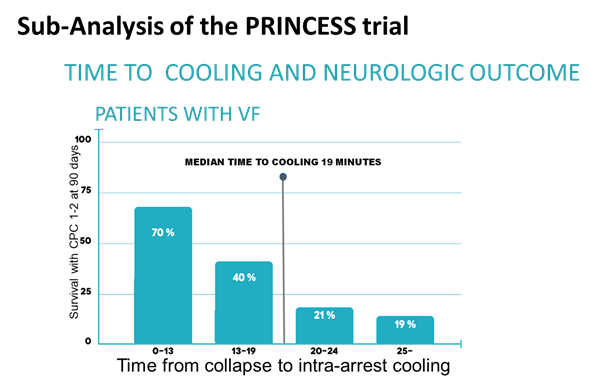
A more recent French randomized trial published on October 2nd, 2019 showed that patients who had been resuscitated from cardiac arrest with nonshockable rhythm, moderate hypothermia (33°C) led to a higher percentage of patients who survived with a favorable neurologic outcome than was observed with targeted normothermia[2]. We believe that these results will force practitioner to consider more use of hypothermia for cardiac arrest patients. According to the recent French trial, the targeted temperature reached after ~7 hours following starting the cooling treatment which this time would be even higher by adding the time from cardiac arrest, transportation and screening (~9-10 hours). Patients with more extensive brain injuries may be easier to cool rapidly because they no longer have the compensatory physiology to “fight the cooling”. RhinoChill® aims to reduce this time to as low as 19 minutes which time-analysis publication mentioned above showed that this would lead to a higher survival and better neurological outcome in SCA patients.
Below table summarize the time between randomization to goal target in the most accepted hypothermia trials:
| Year | Trial | Temperature (C) | Mean time (min) |
| 2002 | HACA | 34 | 360 |
| 2013 | TTM | 33 | 720 |
| 2019 | Hyperion | 33 | 317 |
| 2019 | PRINCESS | 34 | 101 |
This disclosure contains information that BrainCool is obliged to make public pursuant to the EU Market Abuse Regulation (EU nr 596/2014). The information was submitted for publication, through the agency of the contact person, on 2021-06-09 13:36 CET.
For more information
Martin Waleij - CEO
+46 - 733 -93 70 76
E-mail: martin.waleij@braincool.se
About BrainCool AB (publ)
BrainCool AB (publ) is an innovative medical device company that develops, markets, and sells leading medical cooling systems for indications and areas with significant medical benefits within the healthcare sector. The company focuses on two business segments, Brain Cooling and Oncology. BrainCool AB (publ) is based in Lund, Sweden, and its share is listed on Spotlight Stock Market.


