BrainCool AB (publ) update of EU grant (BRAINCELL – combined TTM / thrombectomy treatment)
In April 2020, BrainCool AB received a grant of MEUR 3 with the objective of bringing a combination treatment to market using TTM therapy combined with thrombectomy intervention for stroke patients. The main vision of the project is to bring the product concept, BRAINCELL, the world’s first TTM system that is specifically designed for the needs of stroke patients to the market.
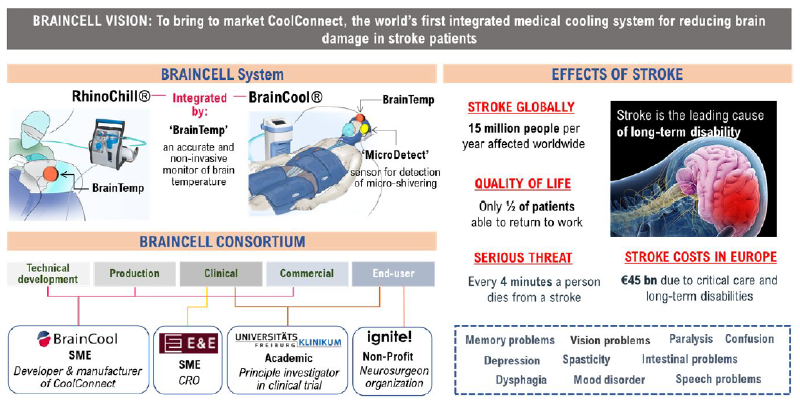
BRAINCELL is a system that can cool brain temperatures in a rapid (as soon as stroke is diagnosed), convenient (does not obstruct the head, chest or limb area during thrombectomy treatment) and sustainable (cooling can extend for over 6 hours) manner, while preventing cold-induced shivering via its unique anti-shivering functionality and high precision temperature control.
Time is brain
The phrase “time is brain” puts emphasis on the fact that, during a stroke, human nervous tissue is rapidly and irretrievably lost, and that therapeutic interventions should be urgently pursued. Compared with the normal rate of neuronal loss in brain aging, the ischemic brain ages 3.6 years each hour without treatment.[1] 15 million people per year are affected by stroke worldwide, thus being a major challenge of the 21st century. Today, up to 50% of the patients remain seriously disabled, caused by brain damage resulting from blocked blood flow, despite undergoing recent advancements in treatments such as intravenous thrombolysis and/or mechanical thrombectomy (MT).
The main vision of the project is to bring the product concept, BRAINCELL, the world’s first TTM system that is specifically designed for the needs of stroke patients, to the market for reducing brain damage that leads to life-changing disabilities. BrainCool has received funding for this EU project amounting to EUR m 3.
- EUR m 2 is primarily designated to the development and industrialisa
tion activities of the RhinoChill® device, but also to projects with the BrainCool System and a new product for non-invasive brain temperature measurement (“BrainTemp”), where a functional protype and IPR has been developed under the completed EU project “BrainSave.” - EUR m 1 is designated for clinical development and trials with this new and unique concept.
The project began November 1, 2020, and this is a summary of the one-year status within the project. The project has used around MEUR 1, i.e. 33% of the total budget during the project´s first year.
Project Background
15 million people per year are affected by stroke worldwide, a third of whom are burdened with permanent handicaps (not able to live without help, loss of memory, loss of speech, loss in use of hands, fingers, legs, droopy face) that are caused by brain damage resulting from blocked blood flow and reduced oxygen supply.
Reperfusion, i.e., the reintroduction of blood flow back into the brain, is a necessary procedure for treatment, however, current methods are under-optimized, and lead to what is known as reperfusion-induced brain injury. As important it is to re-establish blood flow and oxygen supply, as harmful is the resulting of so-called “Reperfusion Injury” - a biochemical cascade, triggered by oxygen, which destroys brain cells. Early hypothermia seems to be the solution to prevent the harmful aftereffects of the reperfusion. This was shown in many studies with sudden cardiac arrest (SCA) patients. In SCA, cooling of the brain prior to reperfusion is a method recommended by guidelines for preventing brain damage. There is a time window for the treatment, and TTM therapy might be lost after the time window of 8 hours. Unfortunately, the recommendation is not currently applicable to stroke, due to the lack of suitable cooling technology.
The BRAINCELL team (see appendix 1), whose expertise ranges from engineering, to manufacturing, neurology, stroke treatment and dissemination, will be working together to change this paradigm by developing the first cooling device that addresses the cooling needs of stroke patients. Stroke-related disabilities is the most common cause of disabilities in Europe and is estimated to cost healthcare systems over EUR m 45 000 per year.
Within the first year, the following milestones have been achieved:
- Inclusion have started of thrombectomy patients in a clinical trial with University of Freiburg of 30 – 40 patients, paving the way for a larger RCT in 2022.
- The final part of the subprojects within a De Novo 510 k process for RhinoChill® System, start of the third-party tests have been started, after a delay of electrical components.
- FDA GMP audit successfully completed, for the production of the single use catheter of the RhinoChill® System paving the way for start of mass production in Q 2, 2022
- Roadmap established for EU MDR approval for a new generation of the RhinoChill® System in 2022, ahead of the pivotal clinical trial in thrombectomy.
- Coolant production process has been validated by the BrainCool team.
For further detailed info of the work of the BRAINCELL (see appendix 1)
CEO Martin Waleij comments:
“I am pleased that we met the milestones of the first year of this EU funded project, that besides of bringing BRAINCELL to the market for stroke, also offers funding and operational support for our devices on the market today in other indications”
“By making BRAINCELL available to neurologists, significant reduction in the incidence and severity of stroke-related disabilities is expected as a result of this project. As MT is now standard of care for acute ischemic stroke and it has been growing in popularity after publication of the landmark trials, there is a significant increase in the proportion of patients receiving MT after 2015, which has translated into reducon of in-hospital mortality and also improvement in disability. However, there is a giant need for further reduction of brain damage, which is what we look into achieving with this project. The creation of a major medical device market in thrombectomy could also lead to several new potential partnerships for BrainCool.”
[1] https://www.ahajournals.org/doi/full/10.1161/01.str.0000196957.55928.ab
This disclosure contains information that BrainCool is obliged to make public pursuant to the EU Market Abuse Regulation (EU nr 596/2014). The information was submitted for publication, through the agency of the contact person, on 2021-10-29 19:40 CET.
For more information
Martin Waleij - CEO
+46 - 733 -93 70 76
E-mail: martin.waleij@braincool.se
About BrainCool AB (publ)
BrainCool AB (publ) is an innovative medical device company that develops, markets, and sells leading medical cooling systems for indications and areas with significant medical benefits within the healthcare sector. The company focuses on two business segments, Brain Cooling and Oncology. BrainCool AB (publ) is based in Lund, Sweden, and its share is listed on Spotlight Stock Market.


