New research article demonstrates production and efficacy potential for novel cVLP/S1-based COVID-19 vaccine
A recent article published in mBio (American Society for Microbiology) illustrates how the cVLP vaccine developed by AdaptVac and University of Copenhagen has successfully been produced using a robust production platform providing scalability, low cost, and safety.
Attana technology has been applied by the team of Prof Ali Salanti at the University of Copenhagen in their development of a COVID-19 vaccine based on the patented Capsid Virus-Like Particle (cVLP) technology. The recently published mBio article is titled, “Two-Component Nanoparticle Vaccine Displaying Glycosylated Spike S1 Domain Induces Neutralizing Antibody Response against SARS-CoV-2 Variants”
The importance of the publication is that it illustrates that an effective cVLP vaccine can be manufactured using a baculovirus-insect cell expression system, which is considered a robust production platform providing scalability, low cost, and safety. Furthermore, by updating the antigen component of the cVLP the technology lends itself to rapid adaptation for new virus variants. The results presented in the article show the cVLP having potent neutralizing responses against both the original SARS-CoV-2 virus (Wuhan) and the Alpha (UK) variant. Attana technology was used to measure the kinetics and affinity of the vaccine to ACE2 receptors in order to validate function of the vaccine and a strong neutralizing capacity.
As previously communicated, Attana AB has no direct financial interests in this project. Our participation is solely based on our long-term relation with Prof. Salanti and his team and our wish to contribute to a solution of the Coronavirus pandemic.
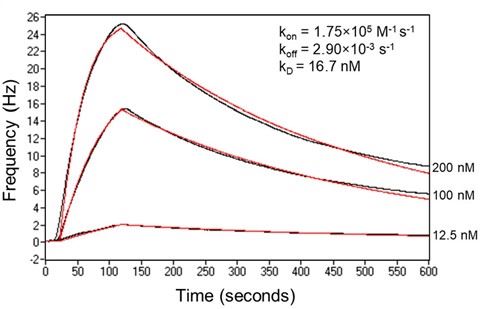
Ace2 binding kinetics: Performed using an Attana Cell™ 200 Biosensor. Expressed hACE2 at 50mg/ml (Expres2ion Biotechnologies) was immobilized on an Attana LNB carboxyl chip. The binding of S1 proteins to the chip was measured in a 2-fold serial dilution series (200 nM to 6.25 nM) in PBS, pH 7.4. Real-time binding (black curves) was fitted in a 1:1 simple binding model (red curves).
For more information, please contact:
Teodor Aastrup, CEO
teodor.aastrup@attana.com
+46 (0)8 674 57 00
The Board of directors for Attana consider that the information in this press release is not likely to have a significant effect on the share price but is of general interest for the shareholders and hence should be communicated.
About Attana
Attana was founded in 2002 with the vision of in vitro characterization of molecular interactions mimicking in vivo conditions. Since then, Attana has developed proprietary label free biosensors for biochemical, crude, sera, and cell-based assays and the Attana Virus Analytics (AVA) platform, a proprietary in vitro diagnostics (IVD) tool. Attana products and research services are used by Big Pharma, biotech companies and academic institutions within the life sciences. To learn more about our latest services and products, please visit www.attana.com or contact sales@attana.com
Tags:


