Getinge launches single-use bioreactor for clinical cell and gene therapies
Empowering researchers with a turn-key solution, Getinge introduces the AppliFlex ST GMP, a cutting-edge single-use bioreactor system designed to seamlessly bridge the gap between research and clinical production in cell and gene therapy as well as mRNA production.
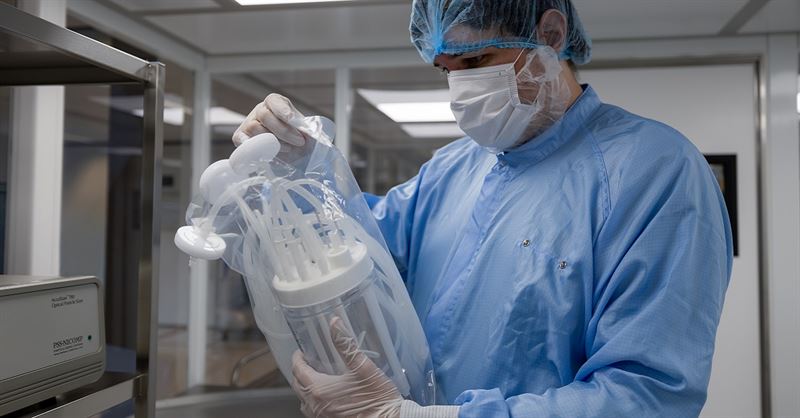
Getinge’s new AppliFlex ST GMP represents a significant milestone in bioprocessing advancement, providing researchers with a seamless turn-key solution for translating bioprocesses from laboratory research to clinical applications. Carefully designed, this single-use bioreactor plays a transformative role in bringing cell and gene therapies to market.
“Built upon the proven success of the stirred tank bioreactor AppliFlex ST, the AppliFlex ST GMP brings scalability and flawless process translation to the clinical stage. Available in different sizes, this bioreactor system provides a comprehensive cGMP manufacturing solution, without compromising in efficiency and ease,” says Sean Herdlein, Vice President Bio Processing at Getinge.
Getinge's solution includes consumables, auxiliaries, verification, and GMP-compliant software, ensuring a well-rounded solution for clinical researchers and producers. Therefore, the AppliFlex ST GMP is suitable to manufacture material for clinical trials and for clinical production.
“With over 40 years of experience in cGMP system qualification, we are proud to expand our portfolio with lab-scale single-use cGMP bioreactors to keep supporting researchers on their path to clinical success,” Sean continues.
Tom van Arragon, Senior Product Manager Life Science at Getinge adds:
“With the addition of a new manufacturing cleanroom for consumables at our Getinge facility in Delft, Netherlands, we are ready to grow with our customers by producing the consumables that meet their manufacturing demands.”
The AppliFlex ST GMP complies with regulations for GMP-compliant research and production environments and sets new standards for innovation and regulatory alignment.
Disclaimer
Purpose/intended use; Getinge’s GMP compliant systems are intended to be used in GMP compliant production and research environments. The system is not intended to be used as a medical device.
Media Contact:
Anna Appelqvist, VP Corporate Communications
Tel.: +46 (0)10-335 5906
E-mail: anna.appelqvist@getinge.com
About Getinge
With a firm belief that every person and community should have access to the best possible care, Getinge provides hospitals and life science institutions with products and solutions that aim to improve clinical results and optimize workflows. The offering includes products and solutions for intensive care, cardiovascular procedures, operating rooms, sterile reprocessing and life science. Getinge employs over 10,000 people worldwide and the products are sold in more than 135 countries.
Tags:


