Interim Report January-March 2017
“New top-line Phase 3 results support the longterm safety and efficacy of CAM2038 in patients with opioid dependence. After completing presubmission meetings with EMA and FDA, market approval applications for CAM2038 are now being finalized.”Business highlights first quarter 2017 · All patients completed treatment in Phase 3 long-term safety study of long-acting buprenorphine depots in opioid dependent patients. · Pre-MAA/NDA meetings held with EMA and FDA for weekly and monthly buprenorphine depots for treatment of opioid use disorder. · Presentation of Phase 2 results for long-



 Chilean company Seawind has chosen Breeze Wind Farm Management system for wind farm monitoring, reporting and analysis.SeaWind is a Chilean company with more than 10 years experience developing, maintaining and operating wind farms projects in Chile and South America. Having developed more than 90 MW in wind and net billing solar projects, SeaWind is projecting a strong increase in the coming years thanks to a 270 MW portfolio investment.
After intensive market research, SeaWind decided to implement Breeze into its developed wind farm, Cerro Coihue, located in a very remote and windy
Chilean company Seawind has chosen Breeze Wind Farm Management system for wind farm monitoring, reporting and analysis.SeaWind is a Chilean company with more than 10 years experience developing, maintaining and operating wind farms projects in Chile and South America. Having developed more than 90 MW in wind and net billing solar projects, SeaWind is projecting a strong increase in the coming years thanks to a 270 MW portfolio investment.
After intensive market research, SeaWind decided to implement Breeze into its developed wind farm, Cerro Coihue, located in a very remote and windy
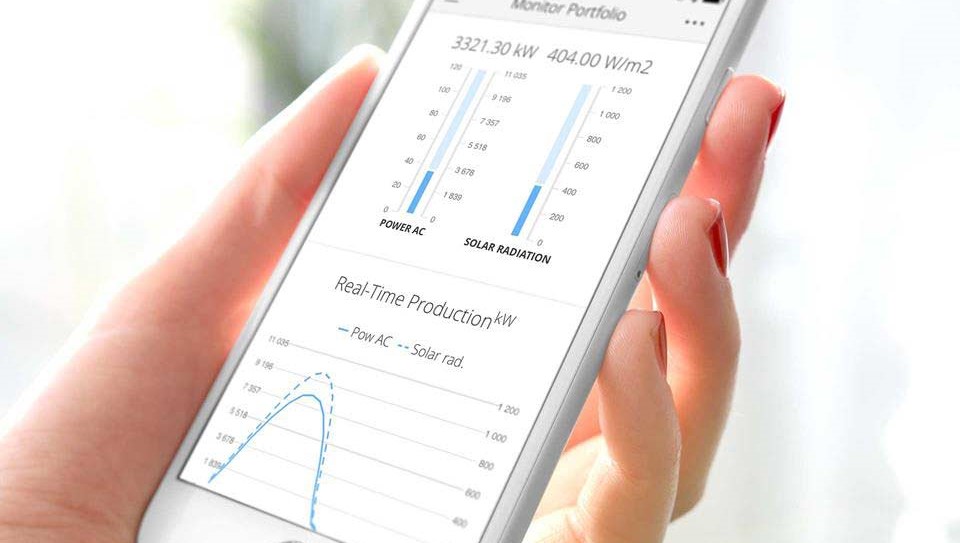 The Breeze and Bright apps brings the most popular features from Greenbyte’s renewable energy management systems to your mobile phone.The apps collect data from your wind farms and solar sites and presents it in an intuitive interface. Access key figures from your entire portfolio and drill down to monitor the status of each site and device on the go.
These updates bring custom alarms to dashboards, app notifications from stops, warnings, and alarms, an updated user interface for Android and a lot more.
· Use the comprehensive overview dashboards to monitor and analyze your
The Breeze and Bright apps brings the most popular features from Greenbyte’s renewable energy management systems to your mobile phone.The apps collect data from your wind farms and solar sites and presents it in an intuitive interface. Access key figures from your entire portfolio and drill down to monitor the status of each site and device on the go.
These updates bring custom alarms to dashboards, app notifications from stops, warnings, and alarms, an updated user interface for Android and a lot more.
· Use the comprehensive overview dashboards to monitor and analyze your