Selumetinib granted orphan drug designation in Japan for neurofibromatosis type 1
30 June 2020 07:00 BST Selumetinib granted orphan drug designation in Japan for neurofibromatosis type 1 Designation follows recent US approval with additional regulatory submissions underway Phase II SPRINT trial showed selumetinib reduced tumourvolume in paediatric patients with NF1 plexiform neurofibromas AstraZeneca today announced that selumetinib has been granted orphan drug designation (ODD) in Japan for the treatment of neurofibromatosis type 1 (NF1), a rare and debilitating genetic disease.[1] Selumetinib is co-developed and co-commercialised with MSD Inc.,

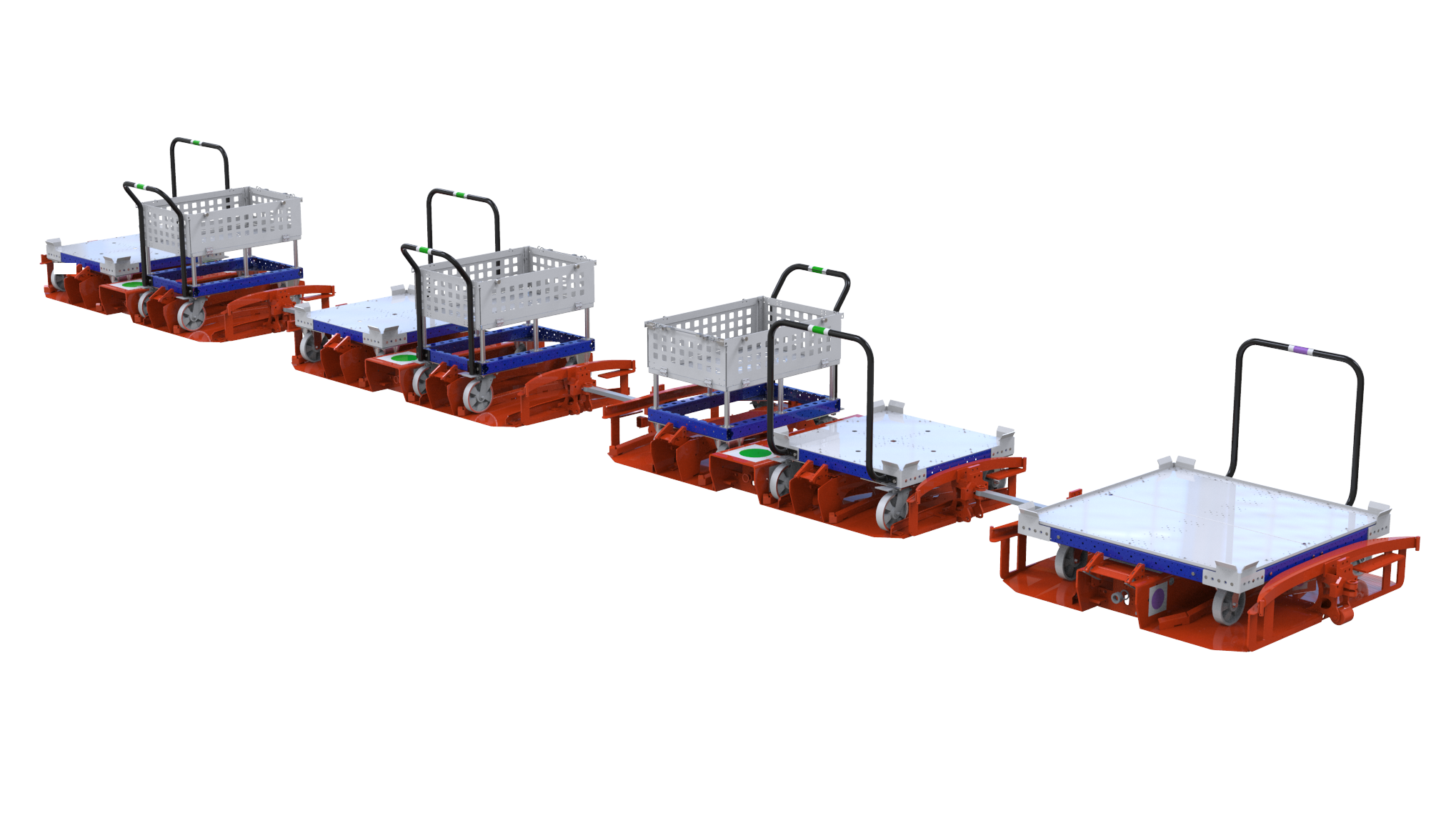 FlexQube has received an order for a German based company, who is setting up a new plant to manufacture automotive parts in Southeast USA, worth 6 million SEK. The order consists of modular FlexQube trolleys in four different versions in combination with the Liftrunner Mother Daughter tugger train system. IPW Lift Techs is managing the deal and FlexQube is selling the products through them. The order will be delivered during the third quarter 2020.
“Once again we win a large tugger train order after showing the customer how strong the combination of the unique and modular FlexQube
FlexQube has received an order for a German based company, who is setting up a new plant to manufacture automotive parts in Southeast USA, worth 6 million SEK. The order consists of modular FlexQube trolleys in four different versions in combination with the Liftrunner Mother Daughter tugger train system. IPW Lift Techs is managing the deal and FlexQube is selling the products through them. The order will be delivered during the third quarter 2020.
“Once again we win a large tugger train order after showing the customer how strong the combination of the unique and modular FlexQube

 Gasum’s new bunkering station for liquefied gas was taken into use in mid-June. The station is located at Port of Nynäshamn, Sweden on the premises of Ports of Stockholm. The new station includes new bunkering solutions enabling ships to bunker environmentally friendly fuel faster than ever.After a year of construction, the new bunkering station is finally ready to serve existing and new customers. It enables bunkering at high speed from two trucks at the same time. The station set-up and the specialized trucks now being used allows bunkering to take place at the same time as unloading or
Gasum’s new bunkering station for liquefied gas was taken into use in mid-June. The station is located at Port of Nynäshamn, Sweden on the premises of Ports of Stockholm. The new station includes new bunkering solutions enabling ships to bunker environmentally friendly fuel faster than ever.After a year of construction, the new bunkering station is finally ready to serve existing and new customers. It enables bunkering at high speed from two trucks at the same time. The station set-up and the specialized trucks now being used allows bunkering to take place at the same time as unloading or

